Chemical and Physical Properties of Molecules
/Master DAT chemical and physical properties with clear explanations of density, solubility, phase changes, reactivity, and high-yield practice questions.
Everything you need to know regarding chemical and physical properties of molecules for the dat
Table of Contents
Part 1: Introduction to chemical and physical properties
Part 2: Structure
a) Polarity
b) Intermolecular forces
Part 3: IR spectroscopy
a) Experimental procedure
b) Key functional groups
c) Example
d) Related forms of spectroscopy
Part 4: NMR spectroscopy
a) Experimental procedure
b) Interpreting an NMR spectrum
c) Splitting and coupling
d) Example
e) C-13 NMR spectroscopy
Part 5: Separation techniques
a) Polyacrylamide gel electrophoresis (PAGE)
b) Extraction
c) Distillation
d) Chromatography
Part 6: High-yield terms
Part 7: Questions and answers
----
Part 1: Introduction to chemical and physical properties
Understanding the chemical and physical properties of molecules helps to make sense of why molecules act the way they do in reactions. There are various methods to determine the properties of molecules. Some properties can be determined by examining the physical structure. Other properties can be examined using spectroscopy or separation techniques.
This guide will go over everything you need to know regarding important topics such as spectroscopy and chromatography. As you study, make note of any bold terms. These are high-yield terms that you need to know for the DAT. When you feel like you have a solid understanding of the concepts in this guide, apply what you’ve learned with DAT-style practice questions.
----
Part 2: Structure
a) Polarity
Polarity is the distribution of electrical charge in a molecule. Polar molecules typically have one or more polar covalent bonds. Atoms that have a greater difference in electronegativity subsequently have more polar bonds.
In terms of organic chemistry, molecules containing only hydrogen and carbon atoms are not polar. This is because hydrogen and carbon atoms have similar electronegativities. Here are a few examples of nonpolar structures:
figure 1: LINE STRUCTURES FOR BUTANE, 2-BUTENE, AND PROPYNE
Each of these structures is nonpolar because their atoms are very similar in electronegativity. When atoms that are more electronegative, such as oxygen, nitrogen, or bromine, are attached to a molecule, they create a bond dipole. Bond dipoles describe the electron density of polar bonds by using partial charges.
Take carbon monoxide for example. Oxygen is much more electronegative than carbon, so it will pull more electron density towards it. Therefore, oxygen will have a partial negative charge, while carbon has a partial positive charge. The bond dipole can be represented by an arrow pointing in the direction of the partial negative charge, with a tail at the end. Additionally, partial positive and partial negative charge signs are shown on the corresponding atom.
FIGURE 2: CARBON MONOXIDE BOND DIPOLE
A molecule can have multiple bond dipoles, such as in water. An H2O molecule has 2 polar bonds, both of which have a bond dipole pointing towards the oxygen atom. These bond dipoles can also be expressed as a molecular dipole, shown by a single arrow that is a vector addition of the other two arrows. The image below depicts this vector addition.
figure 3: WATER MOLECULE BOND DIPOLE AND VECTOR ADDITION
Molecules are polar if they have a net molecular dipole. Here are some examples of polar molecules with the molecular dipole drawn. Also, molecules are usually polar if they contain highly electronegative atoms such as oxygen, nitrogen, and chlorine.
figure 4: BOND DIPOLES FOR MOLECULES WITH ELECTRONEGATIVE ATOMS
Sometimes, a molecule can have polar bonds but not be polar. This typically happens if a molecule has symmetrical polar bonds. Each individual bond dipole cancels the other out, and there is no net or molecular dipole. Take carbon dioxide for example. Both double bonds between the carbon and oxygen atoms are polar and have a bond dipole. Notice that these dipoles pull in opposite directions. If we add them together, they cancel each other out, leaving the molecule with no molecular dipole. Another example is carbon tetrabromide. Each carbon-bromine bond is polar, but the molecule is tetrahedral in shape. The bond dipoles cancel each other out, and the molecule itself is nonpolar.
figure 5: BOND DIPOLES IN NONPOLAR MOLECULES
Another instance relates to the length of a hydrocarbon chain. Take this structure for example. There is an OH attached to one of the ends, creating a bond dipole. The rest of the molecule, however, contains 11 carbon atoms. This lengthy hydrocarbon chain is nonpolar. This molecule, therefore, has a polar region near the OH group, while the rest of it is nonpolar.
figure 6: UNDECANOL LINE STRUCTURE
b) Intermolecular Forces
In addition to the intramolecular forces covered in the previous section, you need to know intermolecular forces. Intermolecular forces are forces between molecules that affect how they interact with each other. There are three main types of intermolecular forces to be aware of for the organic chemistry section. These forces are London dispersion, dipole-dipole, and hydrogen bonding.
London dispersion forces are present in all molecules, but molecules with larger, heavier atoms have stronger dispersion forces. Dipole-dipole forces are found in polar molecules that align the positive end of a molecular dipole with the negative end of a different molecule’s dipole. Hydrogen bonds occur between molecules with hydrogen, as well as oxygen, nitrogen, or fluorine. For a more comprehensive review of intermolecular forces, see our general chemistry guide on liquids and solids.
Intermolecular forces (IMFs) lead to different properties among molecules. Properties such as solubility, boiling point, and melting point are affected by intermolecular forces, and this section will focus on how IMFs affect these properties.
Solubility is the ability of a molecule to dissolve in a solvent such as water. The principle related to solubility is like dissolves like, meaning that polar solvents dissolve polar molecules, while nonpolar solvents dissolve nonpolar molecules. Ethanol, for example, is a polar molecule with an -OH group. Ethanol is a strong solute (compound being dissolved) in other polar solvents such as water. Ethanol and water molecules can hydrogen bond, enhancing the solubility. Propane, on the other hand, is nonpolar. It is not capable of hydrogen bonding, so it will not mix with polar solvents such as water.
Long hydrocarbon molecules with a polar head, such as dodecanol, are not very soluble in water. This is because, even though they have a polar section, the proportion of polar atoms to nonpolar atoms is very small. Therefore, the majority of the molecule is nonpolar, thus making it much less soluble in polar solvents.
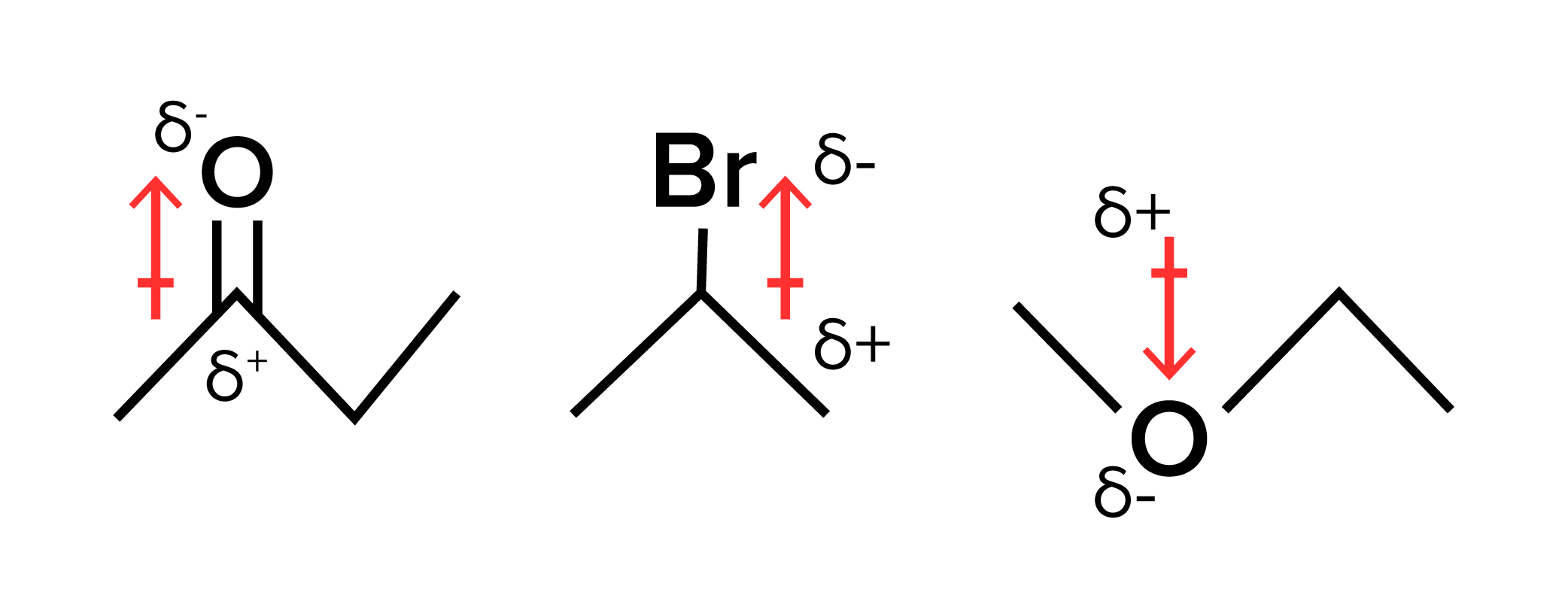
figure 7: ETHANOL, PROPANE, AND DODECANOL
Melting and boiling points of a molecule are also affected by intermolecular forces. In order to melt or boil a compound, energy must be added until a phase change occurs. Stronger or more intermolecular forces translate to more energy needed for a phase change, and therefore a higher boiling and melting point.
figure 8: BOILING AND MELTING POINTS FOR ETHANE, DIMETHYL ETHER, AND ETHANOL
Take these molecules for example. The first molecule, ethane, has the lowest boiling point. Ethane is nonpolar and cannot hydrogen bond, so it only experiences London dispersion forces. The next molecule, dimethyl ethane, has a higher boiling point than ethane. Dimethyl ethane is polar, so it experiences both dipole-dipole and London dispersion forces. Ethanol, the third molecule, is polar and is capable of hydrogen bonding. In addition to hydrogen bonding, ethanol also experiences dipole-dipole and London dispersion forces. Ethanol therefore has the highest boiling point of the three molecules.
Surface area also affects the boiling point of a molecule. Compounds with more surface area can stack easier and therefore have a higher melting and boiling point. If a compound is more branched than a similar, unbranched compound, it will have a lower boiling and melting point. Here are some examples:
figure 9: BOILING AND MELTING POINTS FOR BUTANE, HEXANE, AND OCTANE
As you can see in the compounds above, greater surface area leads to higher boiling and melting points. The compounds below have the same molecular formula, but one of the molecules is branched while the other is not. Isopentane has lower boiling and melting points because it is branched.
figure 10: BOILING AND MELTING POINTS FOR PENTANE AND ISOPENTANE
----
Part 3: IR spectroscopy
a) Experimental procedure
Infrared spectroscopy, often referred to as IR spectroscopy, entails the passage of infrared light through a molecular sample. The energy carried by this light induces vibrational motions that stretch or bend covalent bonds within a molecule.
While the light travels through the sample, a spectrometer gauges the proportion of light that emerges from the opposite end, denoted as percent transmittance, spanning a range of frequencies. The specific frequencies at which the light gets absorbed are determined by the functional groups contained in the sample. Because of this, IR spectroscopy is a powerful tool for identifying particular functional groups and bond types in a compound.
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.