Nonenzymatic Protein Function for the MCAT: Everything You Need to Know
/Learn essential MCAT protein concepts: structural support, motor proteins, immunoglobulins, transport mechanisms, and hormone signaling. Features practice problems and answers.
(Note: This guide is part of our MCAT Biochemistry series.)
Part 1: Introduction
Part 2: Structural proteins
a) Actin and myosin
b) Cytoskeletal elements
c) Kinesins and dyneins
Part 3: Transmembrane proteins
a) GPCRs
b) Pores, ion channels, and active transporters
Part 4: Signaling proteins
a) Peptide hormones
b) Antibodies
Part 5: High-yield terms
Part 6: Passage-based questions and answers
Part 7: Standalone questions and answers
----
Part 1: Introduction
Pop quiz! What is the most abundant protein in our cells? Given how vital enzymatic proteins are to speeding up reactions in our bodies to sustain life, you might guess that the most abundant protein is some sort of enzyme. Hexokinase? PFK? The answer is actually actin.
Actin is a structural protein that helps give cells their shape. In fact, actin is a nonenzymatic protein. Proteins are hugely diverse, and not all proteins have enzymatic properties! Nonenzymatic proteins play an equally important role in our cells as enzymatic proteins. Without them, we wouldn’t survive.
Many unique nonenzymatic proteins co-exist to provide structure and signaling capabilities. While studying nonenzymatic proteins, it’s easy to get them confused. As you work through this guide, it may be helpful to create a chart organizing the different nonenzymatic proteins based on their area of action in a cell and function.
Let’s begin!
----
Part 2: Structural proteins
a) Actin and myosin
How do our bodies and cells maintain their shape? Surely, they’re not just sacs of cytoplasm surrounded by a plasma membrane. There must be something to give our cells and our bodies some structure.
The cytoskeleton and extracellular matrix are compositions of proteins and macromolecules that serve as a scaffold for the cell and tissue, respectively. You can think of the cytoskeleton and extracellular matrix as the framework and foundation of a house; both structures provide support. The cytoskeleton and extracellular matrix are composed of five primary proteins: actin, tubulin, collagen, elastin, and keratin. Actin and tubulin are primarily found within the cytoskeleton of the cell while collagen, elastin, and keratin make up the extracellular matrix.
As noted earlier, actin is the most abundant protein in eukaryotic cells. Inside the cell, actin assembles into long polymers known as microfilaments (shown below). These actin microfilaments possess polarity or a distinction between both ends of the polymer. Just like a magnet has a north and south pole or a battery has a positive and negative terminal, microfilaments have two unique ends.
Figure: An actin microfilament has polarity.
Microfilaments have both a plus (+) end and minus (-) end, which may also be referred to as barbed and pointed ends, respectively. The plus end of microfilaments is where new actin monomers attach to the polymer, while the minus end is where actin monomers dissociate. The association and dissociation of actin monomers is regulated by adenosine triphosphate (ATP). When ATP is bound to actin, the actin monomer will attach to the polymer. As the ATP is hydrolyzed into adenosine diphosphate (ADP), the actin monomer will detach. This constant cycle of actin monomers attaching and detaching is known as treadmilling. This results in an apparent motion of actin strands across a cell’s cytoplasm, which is often useful in cell movement.
Actin also plays an important role in muscle movement. Along with a motor protein called myosin, actin creates movement to contract individual muscle cells. (Recall that a motor protein is a protein that creates movement via the hydrolysis of ATP.) The “heads” of myosin interact with actin filaments in a crossbridge cycle to pull actin close together and shorten the sarcomere, the functional unit of muscle tissue, causing a muscle contraction. Many myosin heads must work together at once to produce enough force to contract a muscle.
Figure: A depiction of the actin-myosin crossbridge cycle.
The first step of the crossbridge cycle involves ATP binding to myosin. When ATP binds to myosin, myosin relaxes its grip to actin and dissociates from the microfilament. Myosin then hydrolyzes ATP into ADP and inorganic phosphate, or Pi. Hydrolyzing ATP causes the myosin head to swing backward and bind to the microfilament. Once myosin releases Pi, myosin starts its power stroke. The power stroke refers to the re-cocking of the myosin head to its original position while attached to the microfilament. Once myosin finishes its power stroke, ADP is released and the cycle can start all over again.
Although each power stroke covers a tiny amount of distance, the distance is amplified by the sheer number of myosin heads and actin filaments in our muscles! You can find additional information on the actin-myosin crossbridge cycle in our guide on the musculoskeletal system.
b) Cytoskeletal elements
In addition to actin, tubulin, collagen, elastin, and keratin work together to form the structure of cells. Inside the cell, alpha and beta forms of tubulin monomers assemble into long polymers called microtubules.
Figure: Microtubules are composed of tubulin.
Microtubule structure is quite different from microfilament structure. The insides of microtubules are hollow. Tubulin monomers associate into heterodimers of alpha-tubulin and beta-tubulin. Due to these heterodimers, microtubules, like microfilaments, have a polar structure. There are both positive and negative ends to microtubules. Alpha-tubulin is exposed at the negative ends of microtubules and tends to be located at the centrosome in the interior of the cell. Beta-tubulin is exposed at the positive ends of microtubules and tends to be located at the edges of the cell, near the plasma membrane. Microtubule polarity is an important feature that dictates the role microtubules play in the cell. We’ll talk more about that role in the next section.
Collagen, elastin, and keratin are largely found in the extracellular matrix. Collagen is a helical fiber made of three interwoven strands and composes a large portion of the extracellular matrix in connective tissue. Collagen provides structure to our tissues, bones, ligaments, and tendons.
Elastin also provides structure to the rest of our body, although it is not quite as abundant as collagen. In their natural state, elastin fibers are long and coiled. When stretched, elastin fibers become more linear in shape while preserving the cross-linked structure of the extracellular matrix. Elastin allows our tissues to stretch and snap back into shape without permanent structural damage.
Keratin is not directly localized in the extracellular matrix but is concentrated in epithelial cells. Keratin provides cells with the necessary structure and stability to protect our bodies and acts as a hard barrier from the outside world. Keratin is also largely found in our fingernails and hair.
c) Kinesins and dyneins
What exactly do microtubules do? Many of the functions microtubules serve are structural. Microtubules provide support so that cells don’t collapse on themselves. Microtubules are vital in mitosis and meiosis to ensure chromosomes are separated.
Recall that microtubules, composed of tubulin, have polarity. This polarity is important because motor proteins—in particular, kinesins and dyneins—use this polarity to walk along microtubules.
Kinesins and dyneins are classified as motor proteins. This means that these proteins move in the cell by hydrolyzing ATP. Scientists have recorded the way kinesins and dyneins move and have theorized that these proteins move in a stepwise manner, similar to how humans walk on two feet. How cool is that?! In fact, kinesins and dyneins move along microtubules. You can think of microtubules as a highway for motor proteins. On a highway, traffic goes in two directions. Similarly, the polarity of microtubules allows kinesin and dynein to travel in opposite directions. Kinesins travel towards the positive end of microtubules (towards the periphery of the cell), while dyneins travel towards the negative end of microtubules (towards the nucleus of the cell).
However, kinesin and dynein aren’t just moving without a purpose. Attached to the heads of kinesin and dynein are usually vesicles or organelles that need to be transported towards the periphery or center of the cell. Kinesin and dynein are like 18-wheeler trucks carrying large cargo from one location to another. These motor proteins are responsible for the bulk of active molecular transport within cells.
Figure: Kinesins and dyneins carry cargo out of or into the cell.
----
Part 3: Transmembrane proteins
a) GPCRs
So far, we’ve seen that nonenzymatic proteins are necessary structural components that facilitate movement in our cells and body. Nonenzymatic proteins also exist in the periphery of cells, primarily the plasma membrane. Nonenzymatic proteins are necessary to transduce exterior signals and allow ions and molecules into the cell.
One such example of a signal-transducing protein system is a G-protein coupled receptor (GPCR). A GPCR is a transmembrane receptor that changes conformation when a ligand binds to it. The conformational change activates a nearby G-protein on the inside of the cell. (The namesake of the G-protein is the GTP or GDP that is typically associated with it.) The G-protein is active when bound to GTP and inactive when GTP is hydrolyzed to GDP. The GPCR is crucial in exchanging GDP for GTP to activate the G-protein.
The G-protein itself is a trimeric protein, consisting of an alpha, beta, and gamma subunit. The alpha subunit is responsible for hydrolyzing GTP in the G-protein, while the beta and gamma subunits can have other functions in the cell. When GTP binds to a G-protein, these three subunits dissociate to execute their functions. The activity of each of these subunits varies, depending on the specific identity of each G-protein. While you don’t need to learn each form of the G-protein, it is important to remember that individual subunits perform different functions.
You may also see GPCRs referred to as 7-transmembrane receptors, as they span the cell membrane with 7 different hydrophobic regions.
b) Pores, ion channels, and active transporters
In addition to GPCRs, nonenzymatic proteins exist in membranes as pores, ion channels, and active transporters. Pores, ion channels, and active transporters serve as passages for the exchange of ions and molecules through the plasma membrane.
One of the most important properties of the plasma membrane is that it is selectively permeable. Hydrophobic and small polar molecules can diffuse through the membrane down their concentration gradient with ease, through a process known as simple diffusion. Larger molecules or more polar molecules must be assisted through the plasma membrane through special structures like pores, ion channels, and active transporters exist.
Pores and ion channels can allow ions and molecules to diffuse down their concentration gradient. Aquaporin is an especially critical pore, which allows water to pass in and out of the cell. Some ion channels can be gated with ligand or voltage-dependent sensors. These ion channels only let molecules pass through if the correct signaling ligand binds or the cell depolarizes to a certain voltage.
Active transporters are proteins that can move ions and large molecules against their concentration gradient. Active transporters rely on ATP hydrolysis for energy to move cargo. Active transporters can move molecules in conjunction with other molecules (through symporters) or with molecules in the opposite direction (through antiporters). Nonenzymatic proteins play a crucial role in controlling the balance of solutes within and outside of the cell.
You can find more information about transporters in our guide on lipids and cell membranes.
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.
----
Part 4: Signaling proteins
a) Peptide hormones
Can you think of where else nonenzymatic proteins may be found?
In addition to nonenzymatic proteins providing structure within the cell and transmembrane proteins embedded in the cell membrane, there are also nonenzymatic proteins circulating in our blood.
Peptide hormones are one such component circulating in our blood, thanks to the endocrine system. A hormone is a slow-acting chemical messenger that travels through the bloodstream. They are a slow—but effective—way of sending messages between organ systems. Hormones come in two forms: steroid hormones and peptide hormones.
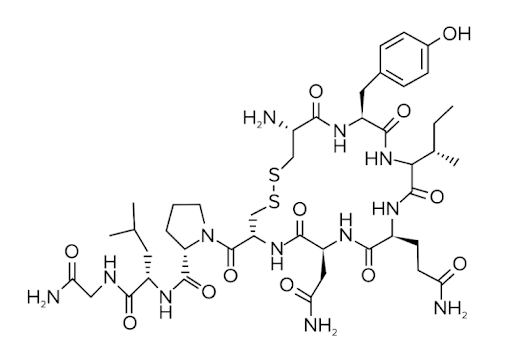
Here, we’ll discuss peptide hormones. They are exactly what they sound like: hormones that are peptides or proteins (shown below). Some examples of peptide hormones are oxytocin, insulin, and follicle-stimulating hormone (FSH). Peptide hormones exert their effect by binding to receptors on cells and causing conformation changes to the receptor. This is one key difference with steroid hormones, which can directly pass through the plasma membrane into the cell.
Figure: Oxytocin is a peptide hormone.
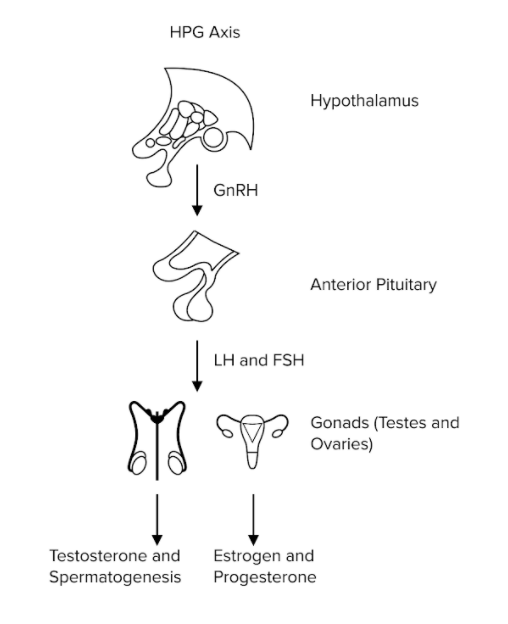
Peptide hormones are particularly important in the HPG axis (shown below), or the hypothalamic-pituitary-gonadal axis. The HPG axis describes the sequential stimulation of hormones from the hypothalamus to the reproductive gonads. The axis begins with the hypothalamus, where gonadotropin-releasing hormone (GnRH), a peptide hormone, is produced. GnRH acts on the anterior pituitary gland, stimulating the release of yet more peptide hormones! The anterior pituitary gland releases luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in our blood. Both LH and FSH have different effects in male and female bodies. In males, LH and FSH are responsible for stimulating testosterone synthesis and spermatogenesis, respectively. In females, LH and FSH are responsible for stimulating estrogen and progesterone production to promote ovulation and maintain the menstrual cycle.
Figure: GnRH, LH, and FSH are peptide hormones in the HPG axis.
You can find more information about the HPG axis and other hormones in our guide on the endocrine system.
The strength of a peptide hormone signal is directly related to its ability to bind to its receptor. This information can be provided in the form of an equilibrium constant for the following reaction:
R + H ↔ RH
Where “R” represents the receptor, “H” represents the peptide hormone, and “RH” represents the bound system. For more information on binding constants, be sure to refer to our guide on enzymes.
b) Antibodies
What happens when we fall sick? We tend to have a fever, drink lots of orange juice, and rest. Why? We try to give our immune system the time it needs to fight off our illness.
You may know that our immune system has two response systems: innate and adaptive. Our innate immune system consists of nonspecific defenses against pathogens and foreign material our bodies encounter. Our adaptive immune system provides us more specific defenses against particular foreign bodies, also known as antigens. The adaptive immune system is able to provide such a specific defense because of antibodies.
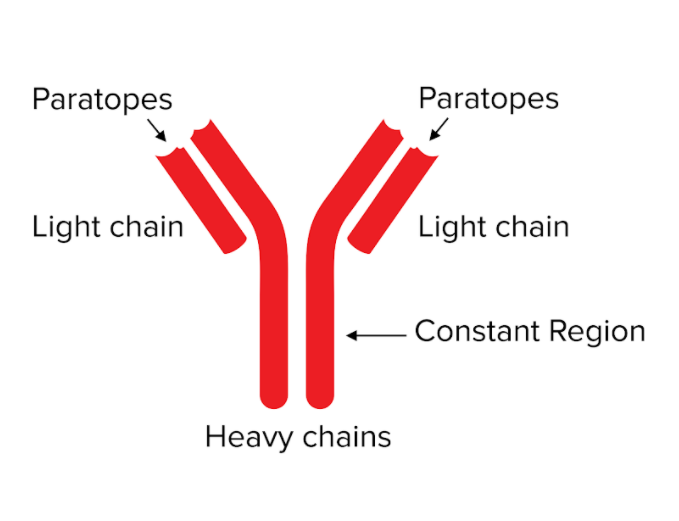
Antibodies, or immunoglobulins, are key to this adaptive response. Antibodies are proteins that circulate in our blood to detect pathogens or foreign material and alert the adaptive immune system. They look like Y-shaped proteins (shown below). Their structure consists of two “heavy” and “light” peptide chains held together by molecular bonds. At the tips of the “Y,” antibodies consist of special sequences known as the variable region, which include paratopes. These variable regions provide the ability to recognize foreign substances and flag them as invasive. The side opposite of the tips of the antibody (the bottom of the “Y”) is known as the constant region. The constant region is important for recruiting other antibodies or immune system cells to help destroy the antigen.
Figure: Antibodies contain distinctive regions.
Antibodies can help neutralize their target through three methods: neutralizing, opsonization, and agglutinating. In these methods antibodies either bond to the antigen preventing it from causing damage, signaling other immune cells to destroy the antigen, or cause the antigen to aggregate, respectively.
You can find more detailed information on antibodies and the immune response in our guide on the immune system.
----
Part 5: High-yield terms
Cytoskeleton: composition of proteins and macromolecules that provides structure for the cell; primarily composed of actin and tubulin
Extracellular matrix: composition of proteins and macromolecules that provide structure for tissue; primarily composed of collagen, elastin, and keratin
Actin: the most abundant protein in eukaryotic cells; actin monomers assemble into long polymers known as microfilaments that possess positive and negative polarity
Myosin: a motor protein that works with actin in a crossbridge cycle to contract muscle cells
Tubulin: structural monomer that assembles into heterodimers of alpha-tubulin and beta-tubulin to form microtubules
Collagen: helical fiber made of three interwoven strands and composes a large portion of the extracellular matrix in connective tissue
Elastin: provides structure to the rest of our body; when stretched, elastin fibers become more linear in shape while still preserving the cross-linked structure of the extracellular matrix
Keratin: not directly localized in the extracellular matrix, but provides cells with needed structure and stability to protect our bodies, and acts as a hard barrier from the outside world
Kinesin: a motor protein that travels towards the positive end of microtubules (towards the periphery of the cell)
Dynein: a motor protein that travels towards the negative end of microtubules (towards the nucleus of the cell)
GPCR: G-protein coupled receptor; a transmembrane signal-transducing protein system
G-protein: a trimeric protein, consisting of an alpha, beta, and gamma subunit. The alpha subunit is responsible for hydrolyzing GTP in the G-protein, while the beta and gamma subunits can have other functions in the cell
Pores: transmembrane structures embedded within the cell membrane that allows small, polar molecules to move down their respective gradients; one such example is aquaporin, which facilitates the diffusion of water
Ion channels: allows charged ions to diffuse through the cell membrane; some ion channels can be gated with ligand- or voltage-dependent sensors
Active transporters: proteins that can move ions and large molecule against their concentration gradient; rely on ATP hydrolysis for energy to move cargo
Peptide hormones: hormones that are peptides or proteins (shown below); transduce signals between organ systems and circulate in the bloodstream
HPG axis: hypothalamic-pituitary-gonadal axis; describes the sequential stimulation of hormones from the hypothalamus to the reproductive gonads
Innate immune system: nonspecific defenses against pathogens and foreign material our bodies encounter
Adaptive immune system: provides us more specific defenses against particular antigens; antibodies are key to this adaptive response
Variable region: includes paratopes on an antibody; sequences of polypeptide that provide the ability to recognize foreign substances and flag them as invasive
Constant region: region on an antibody important for recruiting other antibodies or immune system cells to help destroy the antigens
----
Part 6: Passage-based questions and answers
The mechanochemical cycle of dynein consists of ATP binding and hydrolysis that induces conformation changes within dynein. ATP hydrolysis causes dynein to assume its pre-powerstroke position. ATP hydrolysis also reduces dynein’s affinity for microtubules. When phosphate leaves dynein, dynein rebinds to the microtubule and exerts its powerstroke, moving forward. It is known that at least one ATP must be hydrolyzed for dynein to take a step.
Microtubule-associated proteins are proteins that bind to either end of microtubules, and serve to moderate the assembly of microtubules or regulate velocity. They may also serve to recruit kinesins, dyneins, and other cellular motors. Other examples of MAPS include TPX2, which binds along the lattice structure itself; CLASP, which binds at the plus end; and Patronin, which binds at the minus end.
She1 is a microtubule-associated protein (MAP) found in the eukaryotic cytoplasm. Scientists investigating the role of She1 in dynein motility hypothesize that She1 affects the mechanochemical cycle of dynein. Scientists are able to isolate the She1 MAP through cell lysing, centrifugation, and fractionation of the cell contents. The presence of She1 is confirmed through SDS-PAGE and activity assays.
Question 1: Based on information found in the passage, how does She1 likely affect dynein motility?
A) She1 affects dynein’s ATPase activity.
B) She1 helps dynein by acting like a supporting molecular motor.
C) She1 holds dynein in place during its powerstroke.
D) She1 prevents conformational changes within dynein.
Question 2: Dynein moves towards which polarized end of the microtubule?
A) Positive end
B) Negative end
C) Both positive and negative end
D) Middle of the microtubule
Question 3: Scientists investigating another cytoplasmic protein, Protein X, hypothesize that Protein X affects ATP hydrolysis performed by dynein. How might they test this hypothesis?
A) Scientists could measure the rate of ATP hydrolysis with and without the presence of Protein X
B) Scientists could isolate Protein X and determine whether Protein X hydrolyzes ATP.
C) Scientists could introduce Protein X to kinesin and determine whether it affects kinesin’s ATP hydrolysis.
D) Scientists could introduce Protein X to myosin and determine whether it affects myosin’s ATP hydrolysis
Question 4: Upon further investigation, scientists discover that She1 reduces the stepping frequency of dynein. How might She1 reduce the stepping frequency of dynein?
I. She1 slows down the hydrolysis of ATP by dynein.
II. She1 promotes dynein binding to microtubules.
III. She1 increases the hydrolysis of ATP by dynein.
A) I and III
B) II
C) I and II
D) I, II, and III
Answer key for passage-based questions
Answer choice A is correct. The passage states that She1 is involved in the mechanochemical cycle of dynein. This likely means that She1 affects the physical movement or chemical hydrolysis performed by dynein (choice A is correct). There is no information suggesting that She1 acts as a molecular motor (choice B is incorrect) or that She1 interferes with binding and conformational changes (choices C and D are incorrect).
Answer choice B is correct. Dynein moves toward the negative end of microtubules (choice B is correct) while kinesin moves toward the positive end (choice A is incorrect). The polarity of the microtubule is responsible for the motion of both dynein and kinesin and provides an orientation along all locations on the microtubule (choices C and D are incorrect).
Answer choice A is correct. Scientists are trying to discern how Protein X affects the ATP hydrolysis of dynein. The best approach would be to measure ATP hydrolysis of dynein with and without She1 present (choice A is correct). While measuring ATP hydrolysis activity in other experiments may be useful as a control experiment, the presence of dynein is crucial to test this hypothesis (choices B, C, and D are incorrect).
Answer choice C is correct. A reduced stepping frequency means that dynein will move less often than normal. According to information on the mechanochemical cycle of dynein presented in the passage, we can see that dynein’s movements are directly dependent on ATP hydrolysis and microtubule affinity. Only a lower rate of ATP hydrolysis (I) and increased affinity for microtubules (II) would reduce the stepping rate of dynein. Increasing the rate of ATP hydrolysis would increase the stepping frequency of dynein (III).
Want more MCAT Practice Questions? Check out our proprietary MCAT Question Bank for 4000+ sample questions and eight practice tests covering every MCAT category.
Gain instant access to 4,000+ representative MCAT questions across all four sections to identify your weaknesses, bolster your strengths, and maximize your score. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.
----
Part 7: Standalone questions and answers
Question 1: What important function do actin filaments and myosin play in the cell and human body?
A) Hydrolyze ATP
B) Transduce signals
C) Transport organelles
D) Generate movement
Question 2: Through which processes do antibodies help contain antigens and pathogens?
A) Neutralization, opsonization, and precipitation
B) Neurulation, opsonization, and agglutination
C) Neutralization, opsonization, and agglutination
D) Neutralization, optimization, and aggregation
Question 3: Scientists have been studying the actin-myosin crossbridge cycle. In one experiment, it is noted that myosin successfully binds ATP and releases ADP, but there is no apparent movement of actin microfilaments. Which of the following is a plausible explanation for this phenomenon?
A) A mutation in the myosin causes it to attach to actin microfilaments directly instead of swinging the myosin head backward
B) Myosin is unable to hydrolyze ATP
C) Multiple myosin heads are moving in opposite directions, causing no net movement.
D) Not enough information presented to answer the question
Question 4: How are peptide hormones different from steroid hormones?
A) Peptide hormones are derived from cholesterol while steroid hormones are made of amino acids
B) Steroid hormones act faster than peptide hormones
C) Peptide hormones are synthesized in the nucleus of the cell
D) Peptide hormones exert their effect by binding to receptors while steroid hormones enter the cell
Answer key to standalone questions
1. Answer choice D is correct. Many different proteins and molecules in the cell can hydrolyze ATP and transduce signals (choices A and B are incorrect). Microtubules are the primary cytoskeletal protein involved with transporting organelles and vesicles (choice C is incorrect). Myosin and actin filaments generate movement in skeletal, smooth, and cardiac muscle cells through the actin-myosin crossbridge cycle (choice D is correct).
2. Answer choice C is correct. Antibodies help fight off pathogens in three ways: they bind to the target and render it ineffective, they recruit other immune system cells, or they cause the pathogen to aggregate (choice C is correct).
3. Answer choice A is correct. The question stem states that myosin is able to go through the entire cycle but unable to produce movement. If myosin is not able to swing its head backward, it will not be able to correctly perform its power stroke and move the actin microfilaments despite the presence of ATP hydrolysis (choice A is correct). Without the hydrolysis of ATP, myosin would not be able to release from actin and complete the crossbridge cycle (choice B is incorrect). Due to the polarity of actin, myosin heads can only move in one direction along a microfilament (choice C is incorrect).
4. Answer choice D is correct. The difference between peptide and steroid hormones lies in the way they send their messages. Peptide hormones are unable to pass the hydrophobic portion of the cell membrane, and so must act through cell-surface receptors. Steroid hormones are hydrophobic and can directly pass through cell membranes (choice D is correct). Peptide hormones are derived from amino acids and are thus synthesized in the cytoplasm or endoplasmic reticulum (choice C is incorrect). Steroid hormones are derived from cholesterol precursors (choice A is incorrect). The efficacy of hormone action depends on many other factors in addition to hormone structure (choice B is incorrect).