Thermodynamics for the MCAT: Everything You Need to Know
/Learn essential MCAT thermodynamics concepts, including laws of thermodynamics, spontaneity, and equilibrium, plus targeted practice questions and solutions
(Note: This guide is part of our MCAT Physics series.)
Table of Contents
Part 1: Introduction to thermodynamics
Part 2: Heat
a) Heat capacity and specific heat
b) Heat of transformation
c) Heat transfer
d) Thermal expansion
Part 3: The four laws of thermodynamics
a) Zeroth law of thermodynamics
b) First law of thermodynamics
c) Second law of thermodynamics
d) Third law of thermodynamics
Part 4: Thermodynamic functions, systems, and processes
a) Types of functions
b) Types of systems
c) Types of processes
Part 5: High-yield terms and equations
Part 6: Thermodynamics practice passage
Part 7: Thermodynamics practice problems and answers
----
Part 1: Introduction to thermodynamics
Thermodynamics is an important topic that you likely learned about in chemistry (or physical chemistry) during your undergraduate years. While it is not an extremely high-yield MCAT subject, it can serve as an important foundation for other topics you’ll need to know for the exam.
As you go through this guide on thermodynamics, we’ve bolded several important terms, and we encourage you to create your own definitions and examples as you move through this resource so that they make the most sense to you! At the end of this guide, there’s an MCAT-style thermodynamics practice passage and standalone questions that will both test your thermodynamics knowledge and show you how the AAMC likes to ask questions.
Let’s get started!
----
Part 2: Heat
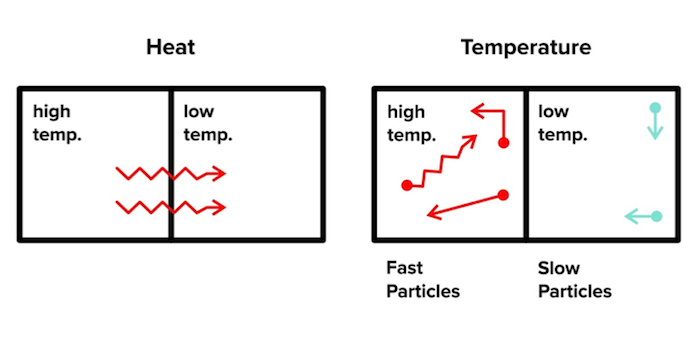
When we talk about thermodynamics, many of the concepts will tie back to an important word: heat. Importantly, heat is not the same as temperature. Let’s take a look at the definitions of each word:
Heat: the transfer of energy based on a temperature difference between two objects
Temperature: a measure of the average energy due to motion of particles in an object (i.e., a cold object has slow-moving particles while a hot object has fast-moving particles)
Figure: Heat vs temperature
Now that we’ve defined heat, let’s dive into some of its properties.
a) Heat capacity and specific heat
Heat capacity is the heat required to raise the temperature of an object by a certain unit of temperature. For example, let’s say our “object” is a chair and we want to raise the temperature by 5 °C. Heat capacity would tell us how much heat we need in order to make that happen.
The SI units for heat capacity are joules (J) / kelvin (K), and this makes sense if we remember that joules are a unit for energy while kelvins are a unit for temperature.
Specific heat is very similar to heat capacity but defines the amount of our “object” and how much we want to raise the temperature of that object. We define specific heat as the amount of heat required to raise one gram of an object by one degree Kelvin (or Celsius). If we go back to the chair example, we now don’t care about the entire chair or raising the temperature by 5 °C. Instead, we want to know how much heat we need to raise one gram of the chair by 1 °K.
b) Heat of transformation
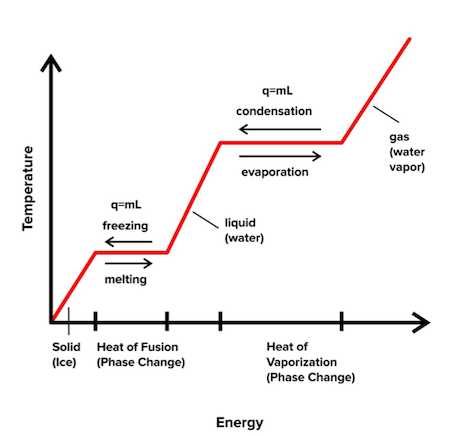
Heat of transformation is another important thermodynamic term. To understand heat of transformation, let’s look at an example. Ice is the solid form of water, and the process of converting ice to water requires heat. Similarly, the process of converting water to water vapor (the gaseous form of water) requires heat. These transitions are called phase changes, and they are often represented by a phase change diagram:
Figure: A phase-change diagram
The phase-change diagram plots temperature versus energy. As we increase the energy, the temperature increases, and we observe a transition from solids to liquids to gases. On the graph, however, you’ll notice flat points in which an increase in energy doesn’t lead to an increase in temperature.
These flat points are the phase changes that we talked about above. During a phase change, the temperature doesn’t change, and we can describe the points with the following equation:
$$q=m\times L$$ $$\mbox{where }q\mbox{ = heat gained or lost from the substance,}$$ $$m \mbox{ = the mass of the substance,}$$ $$L \mbox{ = the latent heat, or } \textbf{heat of transformation,} \mbox{ of the substance}$$
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.