Lipid and Amino Acid Metabolism for the MCAT: Everything You Need to Know
/Explore key MCAT concepts in lipid and amino acid metabolism, with guided practice questions and explanations to maximize preparation and test-day results.
(Note: This guide is part of our MCAT Biochemistry series.)
Part 1: Introduction
Part 2: Fatty acid synthesis
a) The citrate shuttle
b) The oxaloacetate shuttle
c) Palmitic acid synthesis
Part 3: Beta oxidation
a) Lipid absorption
b) Carnitine Shuttle
c) Oxidation of saturated fatty acids
d) Unsaturated fatty acid metabolism
e) Ketogenesis
Part 4: Amino acid metabolism
a) Protein absorption
b) Protein catabolism
c) Urea cycle
Part 5: Metabolic overview and high-yield terms
Part 6: Passage-based questions and answers
Part 7: Standalone questions and answers
----
Part 1: Introduction
Whether you are running a marathon or sleeping in on a Sunday morning, your body is carrying out a plethora of chemical reactions. These reactions all contribute to maintaining homeostasis and using energy. While you may already be familiar with carbohydrate metabolism, your body, the ever-so versatile machine, has additional metabolic pathways to acquire and store energy.
While the MCAT will only rarely test you on details of each metabolic pathway, you will need to understand the big picture behind metabolism by identifying patterns and making connections. Knowing the underlying rationale behind the topics you review is what will ultimately allow you to demonstrate mastery on test day. Make sure to complement your studying with extensive practice, including the practice passage and questions we’ve included at the end of this guide.
Let’s get started!
----
Part 2: Fatty acid synthesis
Fatty acids are long hydrocarbon chains that serve as great sources of energy for the body. The only fatty acid that the human body can synthesize by itself is palmitic acid, a 16-carbon fatty acid.
Figure: Palmitic acid is a 16-carbon long fatty acid.
Its synthesis occurs primarily in the cytoplasm of hepatocytes and follows the net reaction:
7 ATP + 8 Acetyl-CoA + 14 NADPH → Palmitic Acid + 7 ADP + 7 Pi + 8 CoA + 14 NADP⁺ + 6H₂O
The synthesis of palmitic acid is fairly lengthy and is composed of several different components. We’ll walk through each of the stages of this synthesis that you will need to know for the MCAT.
a) The citrate shuttle
After the consumption of excess carbohydrates, acetyl-CoA begins to accumulate in the mitochondrial matrix. Recall that glycolysis produces pyruvate, which is converted into acetyl-CoA via the pyruvate dehydrogenase complex. Citrate synthase then catalyzes the formation of citrate from acetyl-CoA and oxaloacetate.
Typically, this is how acetyl-CoA enters the tricarboxylic acid cycle (TCA). However, since an excess of carbohydrates has been consumed, regulatory measures are taken to slow the TCA cycle, and citrate begins to accumulate. Recall that the TCA cycle’s rate-limiting step is isocitrate dehydrogenase, which acts downstream of citrate synthase—hence causing a build-up of citrate.
To remedy this, citrate is shuttled to the cytoplasm via a citrate shuttle. An enzyme in the cytoplasm catalyzes the reverse reaction of citrate synthase, by splitting citrate into acetyl-CoA and oxaloacetate.
Figure: An overview of the citrate shuttle.
b) The oxaloacetate shuttle
The oxaloacetate that is now present in the cytoplasm then re-enters the mitochondrial matrix in a series of steps:
Oxaloacetate is converted to malate.
Malic enzyme catalyzes the conversion of malate into pyruvate and produces NADPH as a byproduct. This NADPH will be critical in later steps of synthesis.
Pyruvate enters the mitochondrion and is converted into oxaloacetate by pyruvate carboxylase.
Here, oxaloacetate can again be paired with acetyl-CoA to form citrate via citrate synthase.
Why go through the trouble of shuttling citrate and pyruvate back and forth? Note that the oxaloacetate shuttle results in the production of NADPH. This NADPH is a crucial electron carrier that will be needed later in the synthesis. Without the oxaloacetate shuttle, these electrons would not be able to move from the inner mitochondrial membrane into the cytoplasm.
c) Palmitic acid synthesis
In the cytoplasm, acetyl-CoA is converted into malonyl-CoA via the addition of a carbon dioxide molecule. This reaction is catalyzed by acetyl-CoA carboxylase, the rate-limiting step of fatty acid synthesis. Finally, fatty acid synthase, a multienzyme complex, catalyzes the polymerization of palmitic acid. This requires NADPH and produces NADP⁺, carbon dioxide, and water as byproducts.
Figure: Synthesis of palmitic acid.
Note that the synthesis of fatty acids does not require the presence of any precursor or template molecules. This is in contrast to the synthesis of DNA or RNA, which requires the presence of a template molecule to form “copies” of itself.
----
Part 3: Beta oxidation
Beta oxidation (β-oxidation) refers to the breakdown of fatty acids. This process is called β-oxidation because the breakdown occurs at the β-carbon of the fatty acid chain.
Like the synthesis of fatty acids, the breakdown of fatty acids progresses through several steps. First, the fatty acids that are consumed must be digested and absorbed into the bloodstream.
a) Lipid absorption
In the small intestine, lipids undergo chemical digestion by bile and various enzymes, such as lipases. Fatty acids with short chains end up being absorbed into the blood after they cross the intestine. Long-chain fatty acids, however, will form micelles for their absorption and then be assembled into chylomicrons. Chylomicrons are a specific type of lipoproteins that transport dietary lipids, such as triacylglycerols, to tissues. They enter the lymphatic system via lacteals, lymphatic vessels in the small intestine, and re-enter the blood through the thoracic duct.
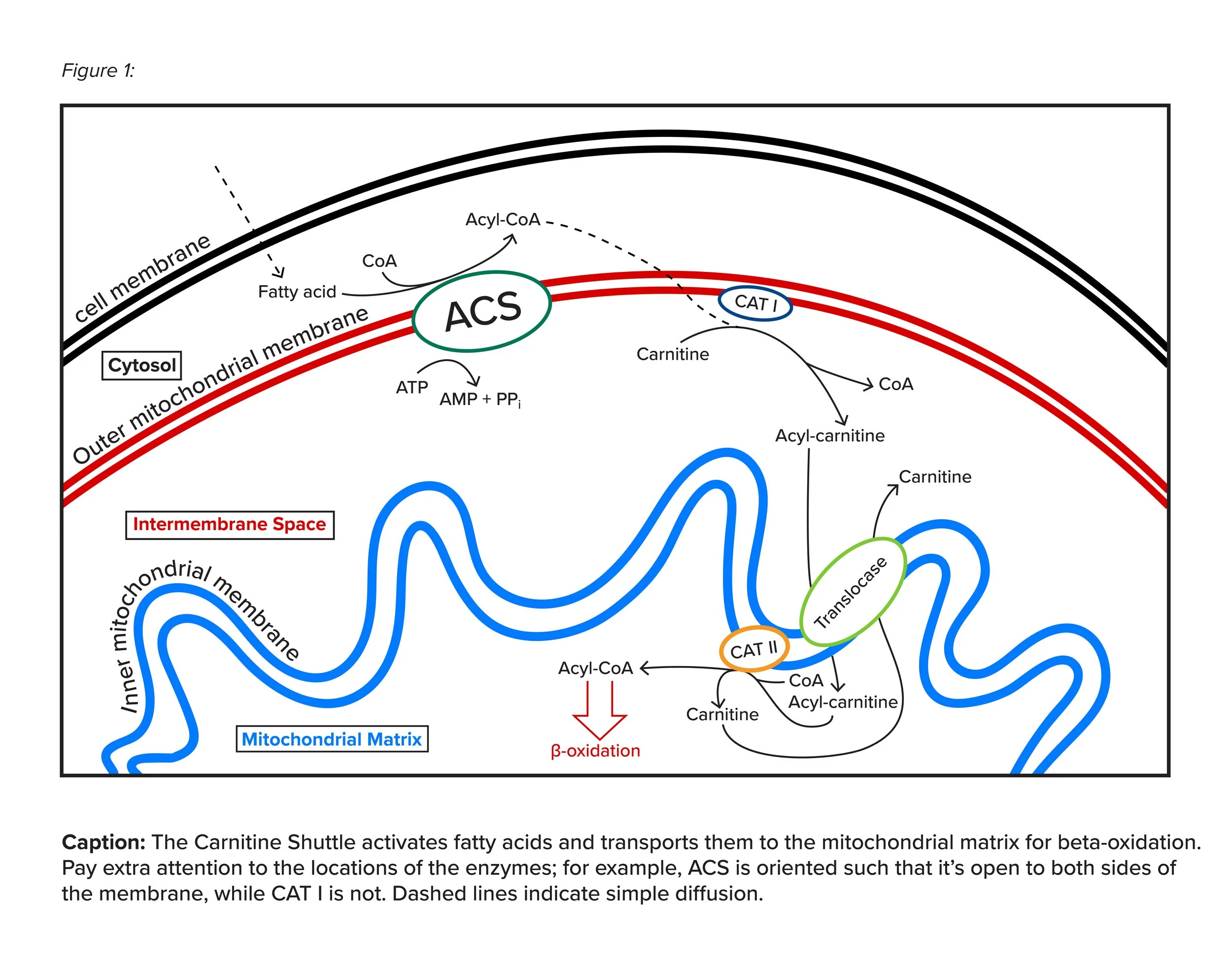
b) Carnitine Shuttle
Before their breakdown, long-chain fatty acids undergo key preliminary processes. They must be transported from the cytoplasm to the mitochondria and activated for beta-oxidation. To do so, they employ the Carnitine Shuttle, which operates in the following mechanism.
Acyl-CoA Synthetase (ACS) uses an ATP to add a Coenzyme A group (CoA) to a cytosolic long-chain fatty acid. This activates the fatty acid and the resulting molecule is known as Acyl-CoA and is the substrate for beta-oxidation. However, it must be transported to the mitochondrial membrane first.
Carnitine Acyl Transferase I (CAT I) then switches the CoA on Acyl-CoA with a Carnitine molecule to make Acyl-Carnitine, bringing the molecule into the intermembrane space. This enzyme marks the rate determining step of the carnitine shuttle.
Translocase sends an Acyl-Carnitine into the mitochondrial matrix and takes a Carnitine (from Carnitine Acyl transferase II in the mitochondrial matrix) out into the intermembrane space in a trade-off-like transfer.
Carnitine Acyl Transferase II (CAT II) switches the Carnitine on Acyl-Carnitine back to a CoA to make Acyl-CoA. You can think of this step as the opposite of transferase I.
Thus, the carnitine shuttle achieves two key goals: activating fatty acids by the addition of a CoA group and transporting them to the mitochondrial matrix, with the end result being an Acyl-CoA molecule ready to undergo beta-oxidation. Note that short and medium chain fatty acids are simply able to diffuse across the mitochondrial membranes due to their nonpolar nature and smaller size, and therefore do not need special transport! Please refer to the figure below for enzyme locations and additional details.
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.
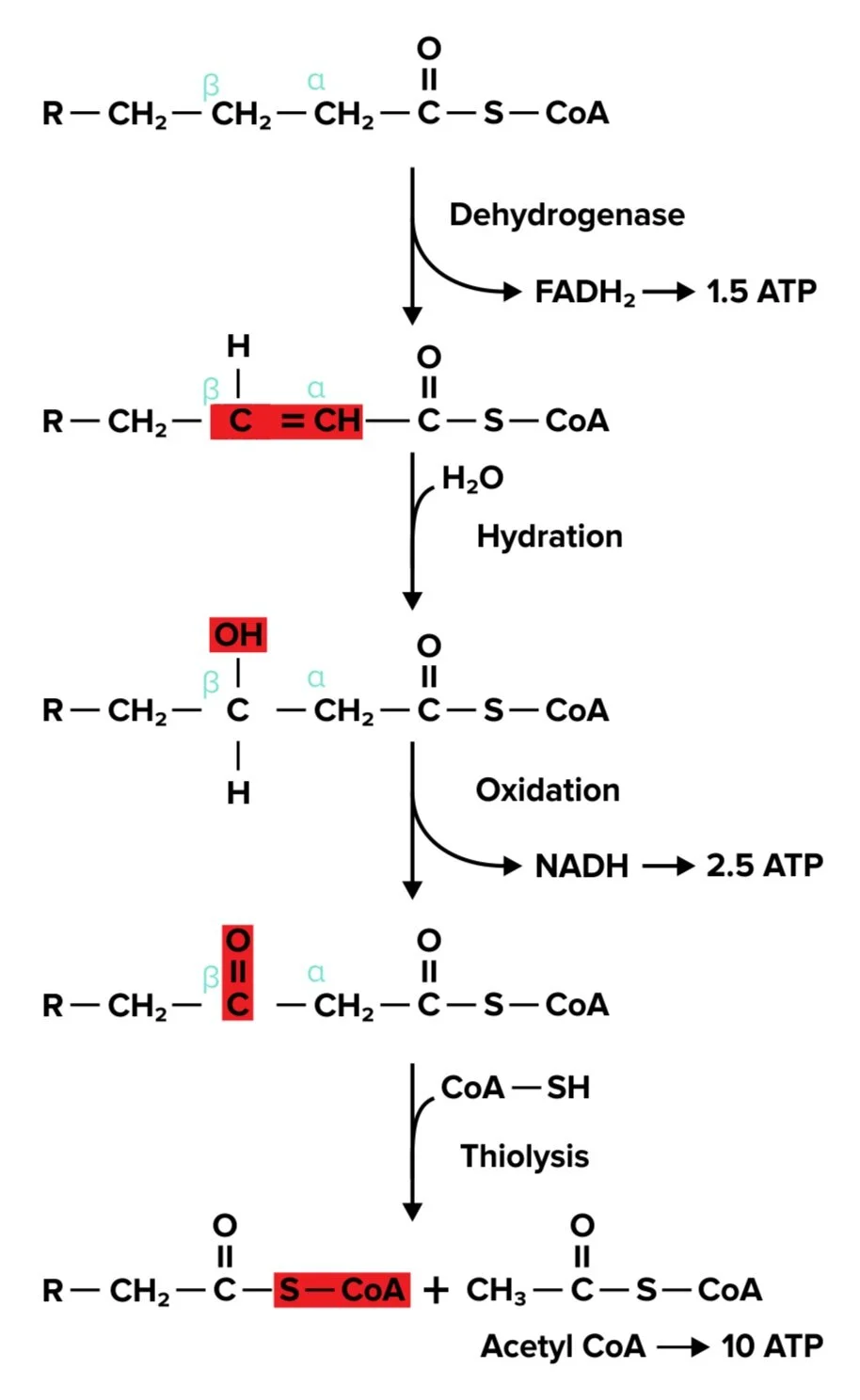
c) Oxidation of saturated fatty acids
Our body can most easily begin to break down saturated fatty acids. Recall that saturated fatty acids possess single bonds between each carbon of the alkyl chain and do not contain any double or triple bonds.
The breakdown of saturated fatty acids, β-oxidation, can be broken down into four basic steps:
Oxidation: A dehydrogenase forms a double bond between the alpha and beta carbons at the end of the fatty acid tail, resulting in the formation of an FADH₂ molecule.
Hydration: A hydroxyl group is added to the beta carbon, breaking the double bond with the alpha carbon.
Oxidation: The hydroxyl group on the beta carbon is oxidized, resulting in the formation of a carbonyl group and the production of 1 NADH molecule.
Thiolysis: Cleavage with CoA-SH results in the formation of acetyl-CoA and the shortened acyl-CoA.
This process is repeated until we are left with one acetyl-CoA, a two-carbon molecule.
While you won’t need to be familiar with each step of this process, it is helpful to understand how long fatty acids are broken down into more useful acyl-CoA molecules.
Figure: An overview of beta-oxidation.
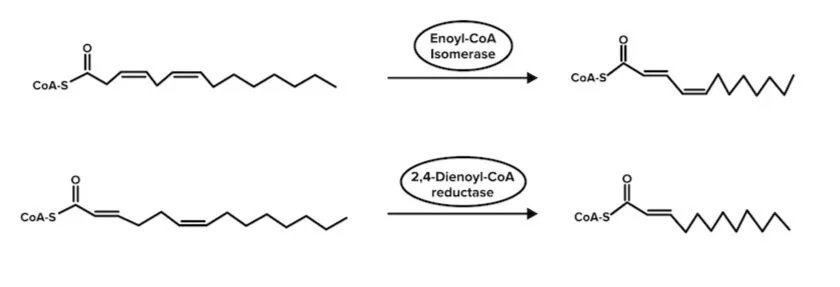
d) Unsaturated fatty acid metabolism
The oxidation of an unsaturated fatty acid is slightly different from that of a saturated fatty acid. It requires additional enzymes to convert the double bonds present in the molecule into single bonds.
For monounsaturated fatty acids, only one additional enzyme is needed. This enzyme will convert the cis double bond between carbons 3 and 4 into a trans double bond between carbons 2 and 3. For polyunsaturated fatty acids, an additional enzyme is used to convert a 2,3 and 4,5 double bond into a double bond between carbons 3 and 4. Then it is converted to a trans double bond between carbons 2 and 3.
Figure: Unsaturated fatty acids must be converted into saturated fatty acids.
Even-numbered fatty acids will yield half as many acetyl-CoA units as the number of total carbons in the original molecule. Similar to how 8 acetyl-CoA molecules are required to produce palmitic acid, a 16-carbon fatty acid, the breakdown of palmitic acid will produce 8 acetyl-CoA molecules.
Odd-numbered fatty acids, on the other hand, vary slightly. Instead of a 16-carbon fatty acid, let's say we have a 17-carbon fatty acid. Now, after cleaving 7 acetyl-CoA molecules, the eighth and final molecule is propionyl-CoA: a 3-carbon molecule. Thus, to calculate the number of acetyl-CoA molecules, subtract 3 from the number of carbons in the original fatty acid, and divide by 2. Our 17-carbon fatty acid gives us 7 acetyl-CoA molecules and 1 propionyl-CoA. This propionyl-CoA will eventually be converted into malate and be fed into the gluconeogenesis pathway. The process by which propionyl-CoA eventually becomes malate is through a primary conversion to succinyl-CoA. Succinyl-CoA can then enter the TCA cycle, where it is ultimately converted into malate. This conversion pathway allows the odd-carbon fatty acid to contribute to gluconeogenesis.
The following formula can be used to calculate the net amount of ATP generated:
12(Acetyl-CoA) + 2.5(NADH) + 1.5(FADH₂) - 2ATP = Net Total ATP
It’s important to note that vitamin B₇, or biotin, is a necessary cofactor for carboxylase enzymes in humans. Thus, biotin is an essential cofactor for fatty acid breakdown.
e) Ketogenesis
During starvation states, the body begins to use specific metabolic processes to meet its energy needs, including gluconeogenesis and glycogenolysis. These processes usually start after 8 - 24 hours of fasting. However, when fasting is prolonged for more than 2 - 3 days and glycogen stores are depleted, ketogenesis kicks in.
During this process, the mitochondria in hepatocytes (liver cells) will convert excess acetyl-CoA into ketone bodies. Ketone bodies are small, water-soluble compounds that can be dissolved in the bloodstream. This allows ketone bodies to pass the blood-brain barrier and cross into the brain—something that fatty acids alone cannot do.
Ketone bodies are utilized by the skeletal muscle and brain. Within these cells, ketone bodies can enter the citric acid cycle and produce the electron carriers NADH and FADH₂.
. These electron carriers can then be utilized in the electron transport chain to produce ATP for the cell.
During times of extended starvation, ketone bodies provide the brain with most of its energy. Note that while the liver initially provides glucose to the rest of the body through gluconeogenesis, the gluconeogenesis pathway consumes oxaloacetate and so cannot continue indefinitely. Ketogenesis and ketone bodies play a critical role in ensuring that the brain and other vital organs are not entirely reliant on gluconeogenesis to meet their energy demands and that there are alternative sources of energy during fasting periods.
----
Part 4: Amino acid metabolism
a) Protein absorption
Carbohydrates, lipids, and proteins each have unique pathways to digestion. In the mouth, salivary amylases initiate the digestion of carbohydrates. Lipids, as we discussed earlier, are primarily digested in the small intestine. Proteins start to undergo proteolysis in the stomach via pepsin. Additional enzymes, such as trypsin, chymotrypsin, and pancreatic juices further break down proteins in the lower digestive tract. In the small intestine, brush-border enzymes finish digestion. These enzymes digest enormous proteins into much smaller amino acids, dipeptides, and tripeptides.
b) Protein catabolism
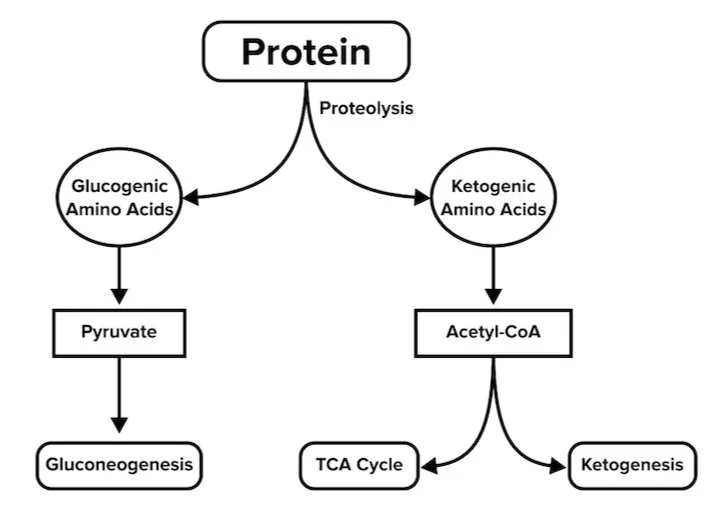
Protein catabolism is the breakdown of proteins and is used only in extreme conditions as a supplement to gluconeogenesis when the body lacks sufficient energy supply. This process generally occurs in the liver and muscle. To begin the process, amino acids first undergo transamination or deamination reactions, which result in the loss of amino groups—either by transferring amino acids to another group or losing it completely.
After the amino acid has lost its amino group, its carbon skeleton can now be converted into glucose or acetyl-CoA. Glucogenic amino acids are converted into pyruvate and will feed into gluconeogenesis to produce glucose. Ketogenic amino acids will be converted into acetyl-CoA, the precursor to ketone bodies.
Figure: Catabolism of amino acids.
c) Urea cycle
When deamination of an amino acid occurs, the amino group is typically released as ammonium—a toxic byproduct! To safely eliminate this toxin from the body, the urea cycle converts ammonium into urea, a compound that can be safely transported to the kidneys for excretion through the urine.
Although knowing every step of the urea cycle is out of scope for the MCAT, you should be aware of its purpose.
----
Part 5: Metabolic overview and high-yield terms
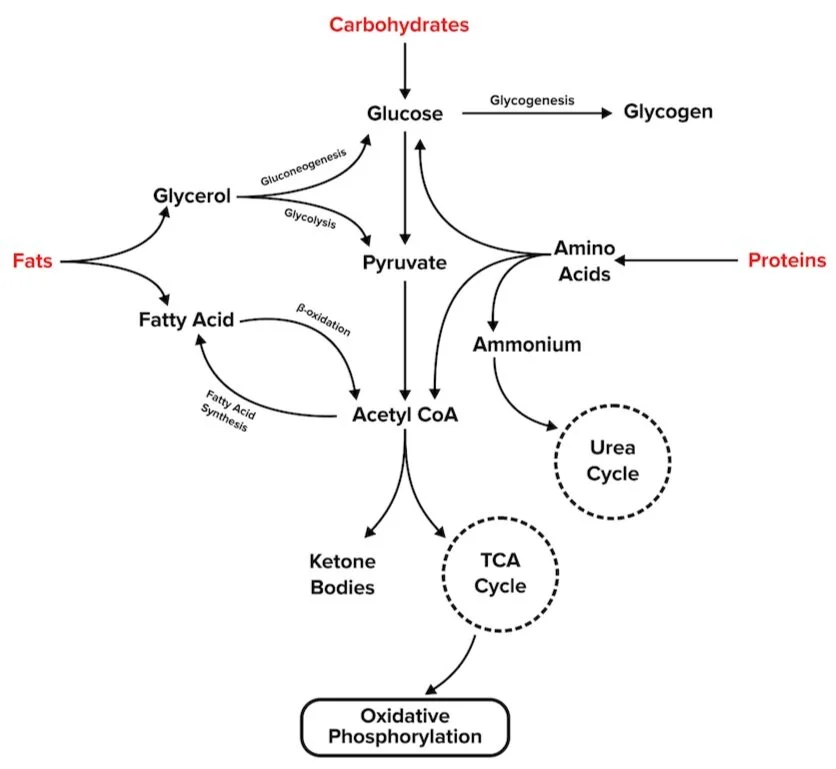
For test day, you will need to understand how metabolic pathways relate to one another. The diagram below illustrates the connection between the metabolism of carbohydrates, proteins, and fats.
Figure: An overview of key metabolic pathways.
Palmitic acid: a 16-carbon saturated fatty acid
Citrate shuttle: a shuttle that moves citrate to the cytoplasm from the mitochondrion
Oxaloacetate shuttle: a shuttle that moves oxaloacetate to the mitochondrion from the cytoplasm
Beta oxidation: the breakdown of fatty acids into acetyl-CoA
Carnitine acyltransferase I: an enzyme in the carnitine shuttle that adds carnitine to fatty acids before entry into the mitochondria
Biotin: vitamin B₇; an essential cofactor for fatty acid breakdown
Ketone bodies: small, water-soluble compounds that can be dissolved in the bloodstream; used by the brain during ketogenesis
Urea cycle: converts ammonium into urea, a compound that can be safely transported to the kidneys for excretion through urine
----
Part 6: Passage-based questions and answers
A common feature of cancer cells is their ability to rewire their metabolism to sustain the production of ATP and macromolecules needed for cell growth, division, and survival. In particular, altered fatty acid metabolism has been found to play an extensive role in cancer pathogenesis. This has resulted in substantial clinical interest in developing therapies that target the reprogramming of fatty acid metabolism. Pharmacological inhibitors of key lipid metabolism enzymes have seen varying degrees of success.
First-generation fatty acid synthase (FASN) targeting drugs, such as C75, orlistat, and cerulenin, initially showed great promise in preclinical studies. In addition to decreasing the synthesis of saturated lipids, they were also found to inhibit tumor growth. However, due to their weak specificity, FASN-targeting drugs indirectly activated carnitine acyltransferase I (CAT1) in peripheral tissue. Next-generation FASN-targeting drugs have been found to display higher specificity with limited off-target effects.
Citrate lyase is an enzyme in the cell cytoplasm that catalyzes the breakdown of citrate into acetyl CoA and oxaloacetate. Inhibitors targeting citrate lyase (ACLY) have also been of interest to researchers due to the increased expression and activity of the enzyme found in several tumor types. Certain ACLY inhibitors, such as ETC-1002, have been found to be well-tolerated and highly effective in lowering LDL cholesterol in patients with cardiovascular disease, thus indicating that ACLY inhibition could represent a well-tolerated therapeutic strategy.
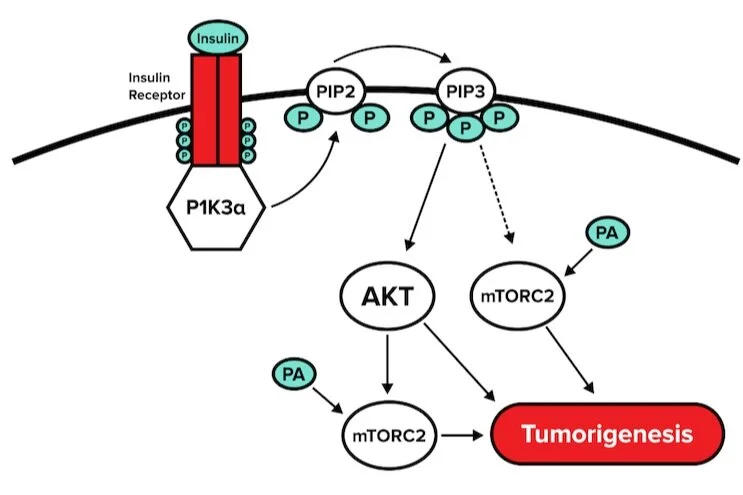
Researchers are also interested in developing dietary interventions to address altered lipid metabolism in cancer cells. It has been demonstrated that a low-carbohydrate, high-fat diet dramatically increases the efficiency of PI3K inhibitors and synergistically reduces the growth of PIK3CA-mutant tumors. Figure 1 illustrates PI3Kα-mTOR signaling.
Figure 1
CREATOR AND ATTRIBUTION PARTY: KOUNDOUROS, N., POULOGIANNIS, G. REPROGRAMMING OF FATTY ACID METABOLISM IN CANCER. BR J CANCER 122, 4–22 (2020). THE ARTICLE’S FULL TEXT IS AVAILABLE HERE: HTTPS://WWW.NATURE.COM/ARTICLES/S41416-019-0650-Z. THE ARTICLE IS NOT COPYRIGHTED BY SHEMMASSIAN ACADEMIC CONSULTING. DISCLAIMER: SHEMMASSIAN ACADEMIC CONSULTING DOES NOT OWN THE PASSAGE PRESENTED HERE. CREATIVE COMMON LICENSE: HTTP://CREATIVECOMMONS.ORG/LICENSES/BY/4.0/. CHANGES WERE MADE TO ORIGINAL ARTICLE TO CREATE AN MCAT-STYLE PASSAGE.
Question 1: The indirect activation of CAT1 by cerulenin may have resulted in which of the following side effects?
A) Weight loss
B) Frequent urination
C) Arterial plaque calcification
D) Hypoglycemia
Question 2: Which of the following is a potential effect of ETC-1002?
A) Accumulation of citrate in the cytoplasm
B) Accumulation of citrate in the mitochondrion
C) Accumulation of acetyl-CoA in the cytoplasm
D) Both B and C
Question 3: Which of the following amino acids could not be a potential phosphorylation site for the insulin receptor shown in figure 1?
A) Threonine
B) Tyrosine
C) Tryptophan
D) Serine
Question 4: Based on the information provided in the passage, why might a low carbohydrate diet reduce tumorigenesis?
A) Low carbohydrate diets would increase blood insulin levels, thus, inhibiting AKT activation
B) Low carbohydrate diets would increase blood insulin levels, thus, inhibiting P1K3α activation
C) Low carbohydrate diets would limit blood insulin resulting in increased AKT activation
D) Low carbohydrate diets would limit blood insulin resulting in decreased activation of P1K3α
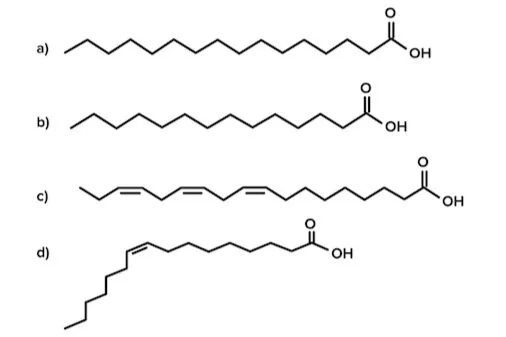
Question 5: The reaction catalyzed by FASN produces which of the following fatty acids?
Answer key for passage-based questions
Answer choice A is correct. CAT1 is an enzyme that catalyzes the rate-limiting step of β-oxidation. Indirect activation of this metabolic pathway would result in the breakdown of fat stores and thus weight loss (choice A is correct). Frequent urination and arterial plaque calcification would not result from the upregulation of CAT1 (choices B and C are incorrect). Hypoglycemia indicates a reduction in the amount of available energy, which would be the opposite result of an upregulation of beta-oxidation (choice D is incorrect).
Answer choice A is correct. As stated in the passage, ETC-1002 is an inhibitor of citrate lyase (ACLY). ACLY catalyzes the breakdown of citrate into acetyl-CoA and oxaloacetate in the cytoplasm. If ACLY is inhibited, acetyl-CoA will not be produced from the reaction (choice C is incorrect). Citrate lyase is not active in the mitochondrion (choice B is incorrect).
Answer choice C is correct. This question does not expect you to know which amino acid residues are phosphorylated in the insulin receptor. Instead, it is testing you on which amino acid residues can be phosphorylated. You should know that only amino acids with hydroxyl (-OH) groups can be phosphorylated. These amino acids include serine, threonine, and tyrosine (choices A, B, and D are incorrect). Tryptophan is not a potential site for phosphorylation (choice C is correct).
Answer choice D is correct. A diet lower in carbohydrates would limit blood insulin levels because insulin release is triggered by the consumption of carbohydrates (choices A and B are incorrect). Figure 1 illustrates an activation cascade that is triggered through the insulin receptor. Downstream of insulin receptor activation is the activation of P13Kα, AKT, and eventually tumorigenesis. A reduction in insulin levels would result in a reduction of P13Kα and AKT activation (choice C is incorrect). Thus, based on the pathway outlined in the figure, low blood insulin levels would result in a decrease in tumorigenesis (choice D is correct).
Answer choice A is correct. Fatty acid synthase (FASN) catalyzes the synthesis of palmitic acid, a 16-carbon saturated fatty acid (choice A is correct). This is the only fatty acid that humans are able to synthesize in the body. Myristic acid is a 14-carbon fatty acid and cannot be synthesized de novo (choice B is incorrect). α-Linolenic acid (ALA) is an 18-carbon polyunsaturated fatty acid (choice C is incorrect). Palmitoleic acid is a 16-carbon monounsaturated fatty acid (choice D is incorrect).
Want more MCAT Practice Questions? Check out our proprietary MCAT Question Bank for 4000+ sample questions and eight practice tests covering every MCAT category.
Gain instant access to 4,000+ representative MCAT questions across all four sections to identify your weaknesses, bolster your strengths, and maximize your score. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.
----
Part 7: Standalone questions and answers
Question 1: What is the rate-limiting step of fatty acid synthesis?
A) Polymerization via fatty acid synthase
B) Acetyl-CoA shuttling via the citrate shuttle
C) Reduction of NADP⁺ during the reaction catalyzed by malic enzyme
D) Conversion of acetyl-CoA into malonyl-CoA via acetyl-CoA carboxylase
Question 2: Which of the following enzymes is involved with the digestion of proteins?
A) Lipase
B) Amylase
C) Trypsin
D) Aromatase
Question 3: What is the purpose of the urea cycle?
A) To catabolize proteins for energy use during extreme conditions
B) To process ammonium to be eliminated from the body
C) To regulate blood pH
D) To generate NADPH for fatty acid synthesis
Question 4: What is the fate of the propionyl-CoA molecule generated by the oxidation of a saturated fatty acid with an odd number of carbon atoms?
A) It undergoes a series of reactions and is ultimately converted to succinyl-CoA
B) It undergoes a series of reactions and is ultimately converted to acetyl-CoA
C) It is marked for destruction by ubiquitin and is degraded by a proteasome
D) It is shuttled to the cytoplasm, where it is degraded by a lysosome
Question 5: Biotin is a key coenzyme for which of the following enzymes?
I. Propionyl-CoA carboxylase
II. Phosphoenolpyruvate carboxykinase
III. Pyruvate carboxylase
A) I only
B) I and II
C) I and III
D) I, II, and III
Question 6: Where does fatty acid synthesis take place in the cell?
A) Mitochondria
B) Ribosomes
C) Cytoplasm
D) Nucleus
Answer key for standalone questions
Answer choice D is correct. Acetyl-CoA carboxylase is an enzyme that catalyzes the rate-limiting step of fatty acid synthesis (choice D is correct). Fatty acid synthase and malic enzyme are both enzymes involved in fatty acid synthesis but are not rate-limiting (choices A and C are incorrect). Similarly, the shuttling of acetyl-CoA is a step in fatty acid synthesis but is also not rate-limiting (choice B is incorrect).
Answer choice C is correct. Trypsin is a key enzyme released by the pancreas to digest proteins. Lipase is involved with the digestion of fats (choice A is incorrect). Salivary and pancreatic amylases work to digest carbohydrates (choice B is incorrect). Aromatase is essential for estrogen synthesis and is not involved with digestion (choice D is incorrect).
Answer choice B is correct. The urea cycle processes the toxic substance ammonium into a safer form, urea, in order to eliminate it from the body (choice B is correct). Protein catabolism, the breakdown of proteins, is used to provide energy during extreme starvation (choice A is incorrect). The bicarbonate buffer system is responsible for regulating blood pH (choice C is incorrect). Malic enzyme catalyzes the reaction that generates NADPH for fatty acid synthesis (choice D is incorrect).
Answer choice A is correct. Recall that in the oxidation of a saturated fatty acid with an even number of carbon atoms, nearly all of the carbon atoms are converted into acetyl-CoA. With an odd-numbered fatty acid, the bulk of the fatty acid is also converted into acetyl-CoA. However, the last 3 remaining carbon atoms on the chain cannot be easily converted into the 2-carbon acetyl-CoA molecule. Instead, the remaining 3-carbon group will undergo a series of reactions to become succinyl-CoA, which can be used in the citric acid cycle (choice A is correct).
Answer choice C is correct. Carboxylase enzymes in humans are biotin-dependent (choices I and III are correct). Phosphoenolpyruvate carboxykinase is an enzyme used during gluconeogenesis to convert oxaloacetate into phosphoenolpyruvate. It is not biotin-dependent (choice II is incorrect).
Answer choice C is correct. Although certain preliminary steps to fatty acid synthesis take place in the mitochondria, such as acetyl-CoA generation, the actual biosynthesis of palmitic acid occurs in the cytoplasm (choice A is incorrect). Ribosomes are the site of protein synthesis (choice B is incorrect). The nucleus stores DNA and is the site of DNA replication and transcription (choice D is incorrect).