Carbohydrate Metabolism for the MCAT: Everything You Need to Know
/A complete guide to carbohydrate metabolism on the MCAT, covering key pathways, enzymes, and practice questions to boost your score.
(Note: This guide is part of our MCAT Biochemistry series.)
Part 1: Introduction
Part 2: Digestion of carbohydrates
a) Enzymatic breakdown
b) Pancreatic regulation
c) Glycogenesis and glycogenolysis
Part 3: Glycolysis and fermentation
a) Glycolysis
b) Lactic acid fermentation
c) Gluconeogenesis
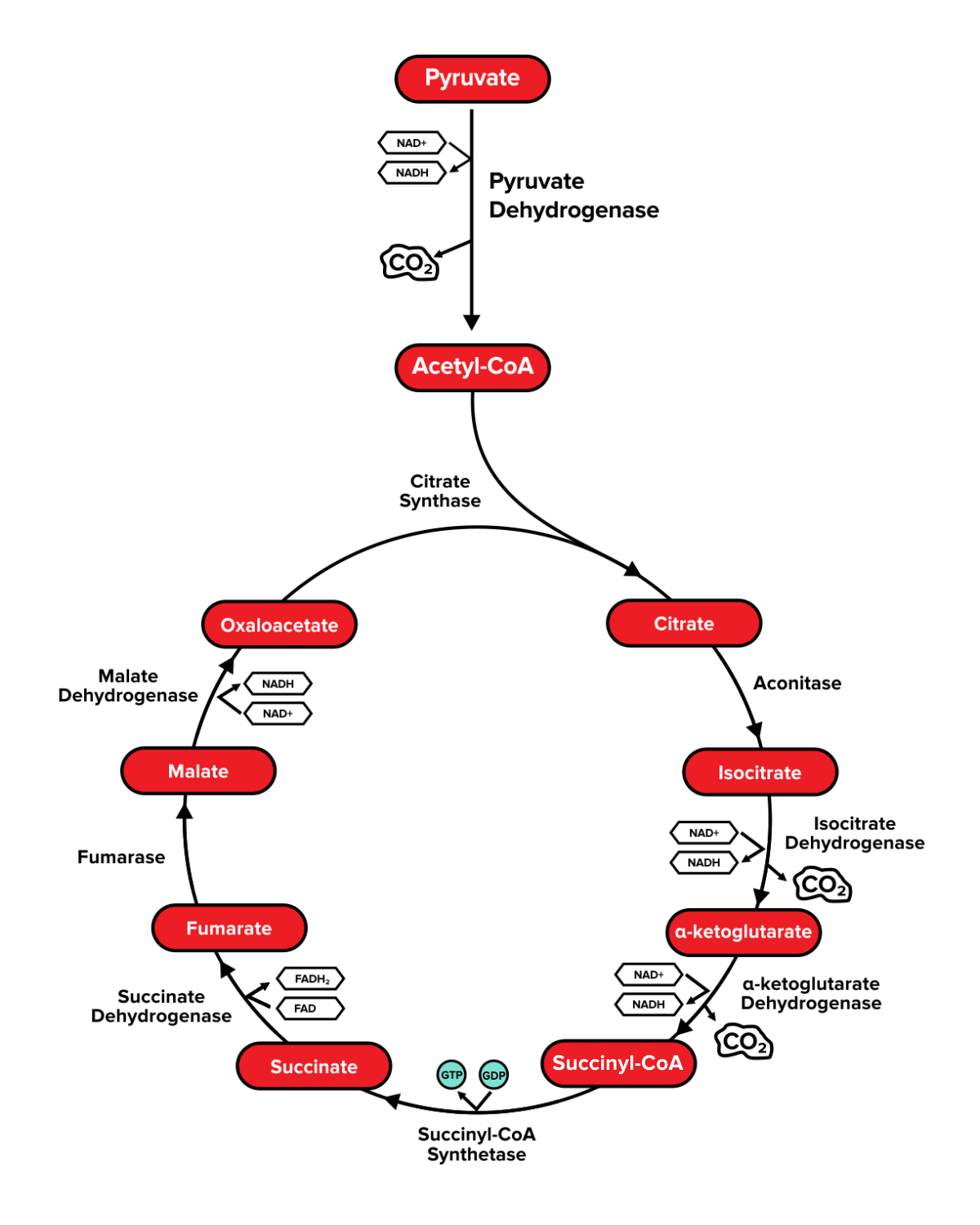
Part 4: Pyruvate oxidation and the TCA cycle
a) Mitochondrial structure
b) Pyruvate oxidation
c) The citric acid cycle
Part 5: Electron transport chain and oxidative phosphorylation
a) The electron transport chain
b) The electrochemical gradient
c) Oxidative phosphorylation
Part 6: Pentose phosphate pathway
Part 7: High-yield terms
Part 8: Passage-based questions and answers
Part 9: Standalone questions and answers
----
Part 1: Introduction
As modernization brings about technological advancements and higher standards of living, it seems to be a double-edged sword. The increased consumption of refined carbohydrates, or highly processed carbohydrates, has become a global problem. In addition to the United States, China and India—two countries that have been lauded for their economic gains these last few decades—are seeing unprecedented levels of obesity and cardiovascular diseases. Thus, it is more critical than ever that healthcare providers understand the biochemical pathways involved with metabolizing the foods we eat and their potential clinical implications.
In this guide, we’ll discuss the digestion and metabolism of one of the MCAT’s favorite macromolecules: carbohydrates. For more information on the carbohydrates and carbohydrates structures themselves, feel free to refer to our guide on carbohydrates.
As we discuss the various pathways involved, make sure to study around the following questions:
When does this pathway occur? (e.g., times of starvation)
Where does this pathway occur? (e.g., the mitochondrial matrix)
Why does this pathway occur? (e.g., to generate energy)
How does this pathway occur? (e.g., what is the mechanism?)
These four questions will help you stay focused on the big picture and not get too lost in the details. At the end of this guide, you will have the opportunity to apply your knowledge with a practice passage and questions.
Let’s get to it!
----
Part 2: Digestion of carbohydrates
a) Enzymatic breakdown
Amylase is the key enzyme involved in the hydrolysis of large polymeric carbohydrates, such as starch, into smaller units. These large carbohydrates can be broken down into monosaccharides and disaccharides: sugar monomers and their linked products.
Amylase can be found in the saliva as salivary amylase and in the pancreatic secretions as pancreatic amylase. Salivary amylase takes action in the mouth, while pancreatic amylase is secreted into the small intestine.
Disaccharidases then further break down the disaccharides in the duodenum. Maltase, sucrase, and lactase break down maltose, sucrose, and lactose, respectively. Thus, we are left with three monomeric carbohydrates: glucose, galactose, and fructose.
b) Pancreatic regulation
In addition to secreting pancreatic amylase, the pancreas is also responsible for the secretion of three essential hormones. These hormones are manufactured by distinct pancreatic cells, which can be found grouped together in structures called islets of Langerhans.
The islets of Langerhans contain three important types of cells: α-cells, β-cells, and δ-cells.
Glucagon is secreted by α-cells when blood glucose levels are low. This hormone promotes both anabolic and catabolic pathways that increase glucose concentration in the blood, such as glycogenolysis and gluconeogenesis.
Insulin is released by β-cells when blood glucose levels are high. It promotes catabolic pathways, such as glycolysis, to derive energy from glucose. It also promotes anabolic pathways, such as glycogenesis, fatty acid synthesis, and peptide synthesis.
Somatostatin is released by δ-cells in response to high blood glucose and amino acid levels. It inhibits the secretion of insulin and glucagon.
For information on additional important roles of the pancreas, refer to our guide on the endocrine system.
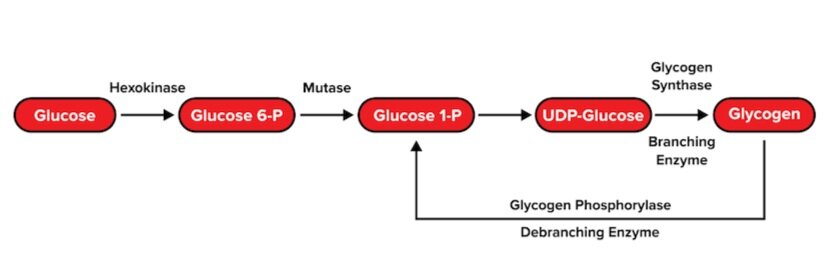
c) Glycogenesis and glycogenolysis
Recall that the body stores glucose monomers in the form of glycogen: a large, branching polysaccharide. To form glycogen, glucose monomers are polymerized via glycogenesis. This process occurs through multiple steps:
Each glucose 6-phosphate monomer is converted into glucose 1-phosphate.
A biomolecule known as uridine diphosphate (UDP) is attached to the glucose molecule.
The glucose monomer is either added to a protein called glycogenin to initiate a glycogen chain or added to a growing glycogen chain by glycogen synthase. Uridine diphosphate is recycled. Glycogen synthase connects glucose monomers linearly using α-1,4 glycosidic linkages.
At certain points, an enzyme known as branching enzyme hydrolyzes one of these linkages. This breaks off an oligosaccharide that the enzyme uses to start a new branch with an α-1,6 glycosidic linkage.
Glycogen is broken back into its glucose monomers via glycogenolysis. Similar to glycogenesis, there are two critical enzymes that will catalyze the reverse reactions of glycogenesis.
An enzyme known as glycogen phosphorylase breaks the α-1,4 glycosidic linkages in a linear chain until it reaches the branching point.
Debranching enzyme hydrolyzes the α-1,4 glycosidic linkage and relocates the resulting oligosaccharide to the end of another linear chain.
Debranching enzyme then hydrolyzes an α-1,6 glycosidic linkage between the branched glucose molecule and the linear chain, resulting in the release of a single glucose monomer.
Figure: Glycogenesis and glycogenolysis.
----
Part 3: Glycolysis and fermentation
a) Glycolysis
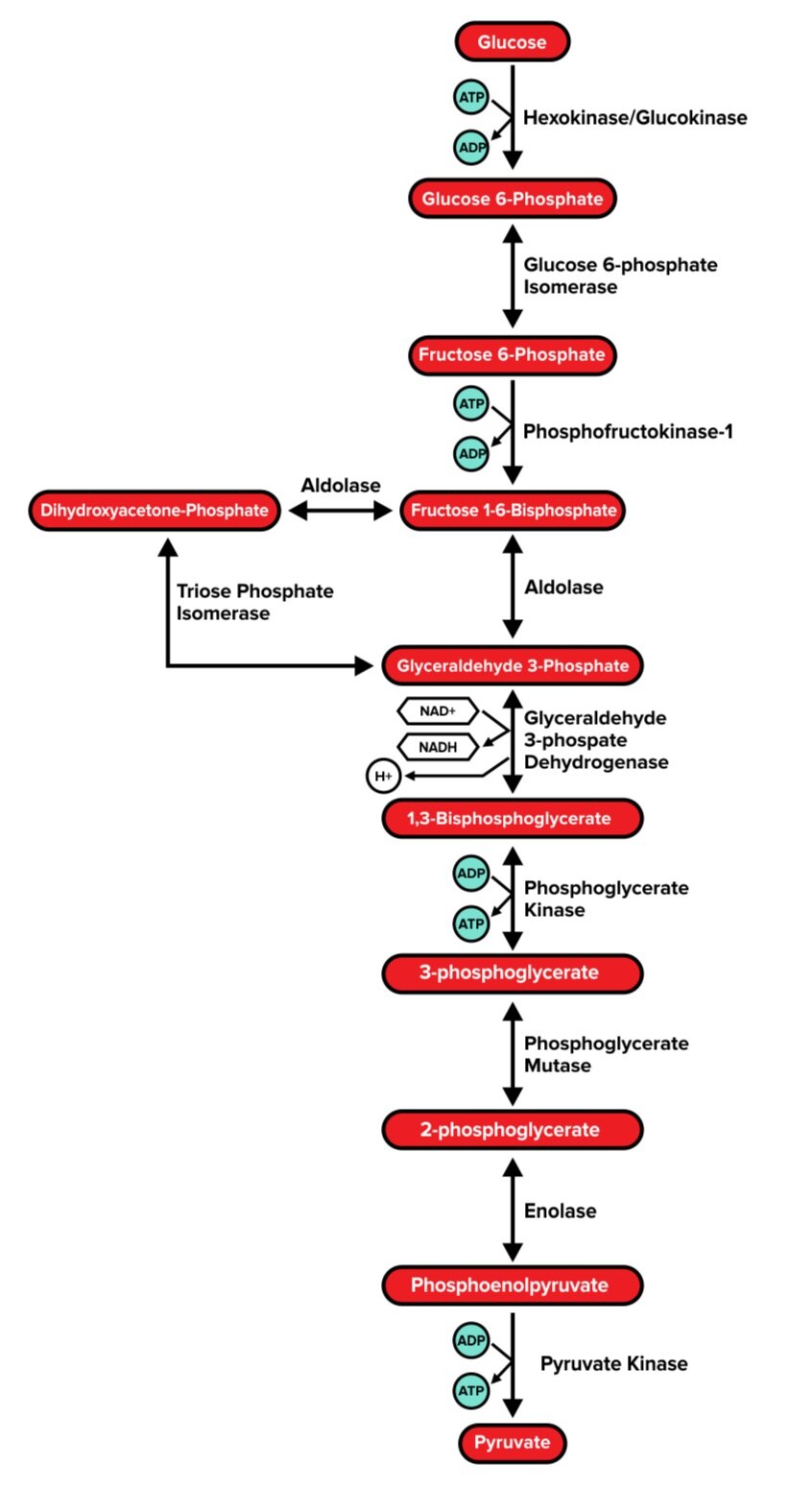
Glycolysis is the process by which a glucose molecule is converted into two molecules of pyruvate. It typically occurs in the cytoplasm. In addition to 2 pyruvate molecules, each glucose molecule that undergoes glycolysis will also result in the production of 2 NADH and 4 ATP molecules. However, during the process, 2 ATP molecules are consumed. Thus, the net products of glycolysis are 2 pyruvate molecules, 2 NADH, and 2 ATP. (These NADH molecules will be quite useful as electron carriers in the electron transport chain, which we will discuss later.)
The following diagram illustrates every step of glycolysis; however, only a handful of these are particularly high yield. While you won’t need to memorize each step of glycolysis and its related enzymes, it may be useful to be familiar with the function of each enzyme.
Figure: An overview of glycolysis. Note that one molecule of glucose (a 6-carbon molecule) yields two molecules of pyruvate (a 3-carbon molecule).
Step 1: Hexokinase/Glucokinase
Glucokinase is found in hepatocytes (liver cells) and pancreatic β-islet cells. It is activated by insulin. Hexokinase, on the other hand, is a bit more universal and found in most tissues. Both enzymes serve the same function: to use ATP to catalyze the irreversible phosphorylation of glucose.
The product of this reaction, glucose 6-phosphate, is now unable to spontaneously diffuse out of the cell. Glucose 6-phosphate also has an inhibitory effect on the hexokinase enzyme.
Step 3: Phosphofructokinase 1 (PFK-1)
Phosphofructokinase 1, also known as PFK-1, catalyzes the rate-limiting step of glycolysis. It uses ATP to catalyze the irreversible conversion of fructose 6-phosphate into fructose 1,6-bisphosphate. This step is highly regulated. Citrate (a metabolic product of aerobic respiration) and ATP have a negative feedback effect on PFK-1.
Why would this be? The presence of citrate and/or ATP indicates that the cell’s energy needs are being met, and thus signals that the glycolysis pathway is not immediately needed. Since this step is an irreversible conversion—and thus requires energy to be performed—shutting down PFK-1 when it is not needed allows the cell to conserve valuable energy.
On the other hand, the presence of AMP (adenosine monophosphate) indicates low energy in the cell and activates PFK-1.
Step 6: G3P dehydrogenase
G3P dehydrogenase catalyzes the reversible conversion of glyceraldehyde 3-phosphate into 1,3-bisphosphoglycerate, which generates one molecule NADH. However, one molecule of glucose (a 6-carbon structure) generates 2 molecules of glyceraldehyde 3-phosphate—so this step yields two molecules of NADH per glucose molecule.
Step 7: Phosphoglycerate kinase
Phosphoglycerate kinase catalyzes the reversible conversion of 1,3-bisphosphoglycerate into 3-phosphoglycerate, or the removal of a phosphate group from 1,3-bisphosphoglycerate. This generates one ATP per molecule of phosphoglycerate (or 2 ATP per glucose molecule).
Step 10: Pyruvate kinase
The final enzyme of glycolysis, pyruvate kinase, catalyzes the irreversible conversion of phosphoenolpyruvate into pyruvate, or the removal of a phosphate group from phosphoenolpyruvate. This generates one ATP per molecule of phosphoenolpyruvate (or 2 ATP per glucose molecule).
b) Lactic acid fermentation
Under anaerobic conditions, or when there is a lack of oxygen, the pyruvate molecules generated by glycolysis will undergo fermentation. During this process, lactate dehydrogenase catalyzes the conversion of pyruvate into lactate (another 3-carbon molecule) and generates NAD⁺ as a byproduct. Since it is the only enzyme in the process, it is the rate-determining step.
Why would our cells perform lactic acid fermentation, if it does not yield any ATP? The primary purpose of lactic acid fermentation is to replenish the NAD⁺ that was converted into NADH during glycolysis by glyceraldehyde 3-phosphate dehydrogenase. This makes additional NAD⁺ available to glycolytic enzymes, so our cells can continue producing 2 ATP at a time through glycolysis.
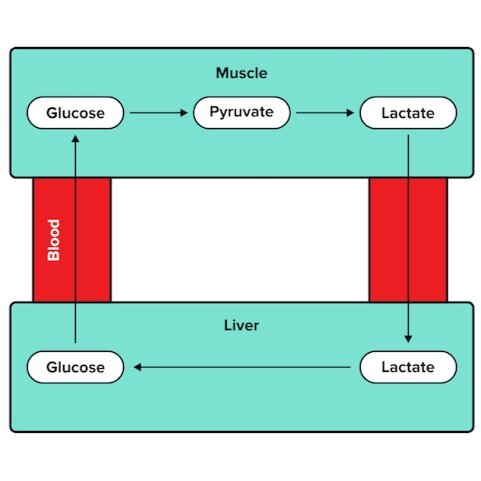
Lactic acid fermentation is part of a larger pathway known as the lactic acid cycle, or Cori cycle. Lactate generated by the muscles is sent to the liver through the bloodstream. The liver has specialized enzymes that can convert lactate into glucose, which is then sent back to the muscles.
Figure: The Cori cycle allows the recycling of lactate.
c) Gluconeogenesis
Between energy stores available in glycogen and dietary intake, the glucose content in the body is usually sufficient to meet energy needs. However, these sources of energy can easily run out during exercise or periods of fasting, for example. How does energy continue to be supplied?
Gluconeogenesis is a metabolic pathway that uses precursors from other sources, for instance, lipids or amino acids, to create glucose. The process can be thought of as the “reverse” of glycolysis: after converting these precursor molecules into pyruvate, several of the same enzymes used in glycolysis will run the reverse reaction to create glucose.
Any reactions that are rate-limiting steps in glycolysis require an additional set of enzymes to catalyze the reverse reaction during gluconeogenesis. Each of these steps requires an additional ATP molecule to proceed spontaneously.
| Glycolytic enzyme | Gluconeogenesis enzyme | Resulting reaction |
|---|---|---|
| Glycolytic enzyme | Gluconeogenesis enzyme | Resulting reaction |
|---|---|---|
Figure: Rate-limiting steps of glycolysis and gluconeogenesis.
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.
The gluconeogenesis pathway is stimulated by the hormone glucagon and inhibited by insulin. For more information on the action of these hormones, be sure to refer to our guide on the endocrine system.
----
Part 4: Pyruvate oxidation and the TCA cycle
a) Mitochondrial structure
Colloquially known as the powerhouse of the cell, the mitochondria play an essential role in metabolism. Under aerobic conditions, the pyruvate formed during glycolysis will enter this organelle for further processing. It's crucial that you know the different structural components of the mitochondrion.
Figure: A simplified view of mitochondrial structure.
The outermost layer of the mitochondrion, the outer membrane, acts as a barrier between the cytosol and the organelle's interior. To serve this function, it is highly impermeable to the passage of ions. The inner layer, or the inner membrane, is the site of the electron transport chain (we’ll discuss this more later in this guide). The inner membrane has many folds called cristae, which increase surface area. The inner mitochondrial membrane is also highly impermeable to ion exchange.
The space enveloped by the inner mitochondrial membrane is known as the mitochondrial matrix. Finally, in between the inner membrane and the outer membrane is the intermembrane space, another important site for the electron transport chain. These regions and their acidity, or hydrogen ion concentration, will become exceedingly important for understanding how the electron transport chain functions.
b) Pyruvate oxidation
Recall that under aerobic conditions, the pyruvate molecules generated by glycolysis will enter the mitochondria. In the mitochondrial matrix, an enzyme known as pyruvate dehydrogenase catalyzes the irreversible conversion of pyruvate into acetyl-CoA and generates NADH and carbon dioxide as byproducts. This enzyme is subject to negative feedback as its product, acetyl-CoA, will inhibit its activity.
The pyruvate dehydrogenase enzyme is not classified as part of glycolysis, nor as part of the citric acid cycle. Rather, it is an intermediate enzyme that catalyzes a transitionary step between the two pathways.
c) The citric acid cycle
In the mitochondrial matrix, acetyl-CoA will enter the citric acid cycle, also known as the Krebs cycle. Although this process does not use oxygen, it is considered aerobic. Why is this?
The citric acid cycle needs both electron carriers—NAD⁺ and FAD—to continue producing NADH and FADH₂. NAD⁺ and FAD are regenerated at a later point in the pathway—during the electron transport chain—by donating hydride ions to an oxygen atom, ultimately forming water. Without the presence of oxygen, the citric acid cycle cannot receive NAD⁺ and FAD, and will ultimately grind to a halt.
For the MCAT, you should be able to recognize the names and functions of the enzymes that carry out this process. The mnemonic "Can I Keep Selling Seashells For Money, Officer" is often used to memorize the TCA cycle's reactants. One enzyme that is particularly important is isocitrate dehydrogenase, as it catalyzes the rate-limiting step of the TCA cycle.
Instead of memorizing the exact structure of every intermediate molecule in this pathway, simply memorizing the number of carbons can help you recognize structures if presented on your exam. A helpful trick is to remember that the number of carbons present on a molecule can help you recall their name. Citrate and isocitrate have 6 carbons each. Alpha-ketoglutarate has 5 carbons. Succinyl-CoA, succinate, fumarate, malate, and oxaloacetate each have 4 carbons. Acetyl-CoA has 2 carbons.
Figure: An overview of the TCA cycle.
Ultimately, each acetyl-CoA molecule that undergoes the TCA cycle will result in the production of 1 GTP, 3 NADH, 1 FADH₂, and 2 CO₂. Since one molecule of glucose results in two acetyl-CoA molecules, the TCA cycle will produce 2 GTP, 6 NADH, 2 FADH₂, and 4 CO₂ for every glucose molecule.
There are three TCA enzymes that are highly regulated. The table below summarizes their activators and inhibitors.
| Enzyme | Activator(s) | Inhibitor(s) |
|---|---|---|
Figure: Highly regulated enzymes of the citric acid cycle.
----
Part 5: Electron transport chain and oxidative phosphorylation
a) The electron transport chain
Remember the molecules of NADH that were generated during glycolysis and the citric acid cycle? Electron carriers such as NADH and FADH₂ have their time to shine at the electron transport chain, a series of redox (reduction-oxidation) reactions that create an electrochemical gradient across the mitochondrion's intermembrane space.
Although these electron carriers are crucial to the electron transport chain, there is one obstacle: NADH and FADH₂ cannot cross the mitochondrial membrane from the cytoplasm. Thus, the cell must use shuttle systems to ferry the electron equivalents into the mitochondria; these shuttle systems include the glycerol 3-phosphate (G3P) shuttle and the malate-aspartate shuttle.
The electron transport chain consists of the reactions catalyzed by four membrane-bound complexes located in the mitochondrion's inner membrane. Several of these complexes belong to a class of enzymes called flavoproteins, which use FAD embedded within their structure to accept and transfer electrons. We’ve listed these first four complexes below. While you won’t need to memorize these complexes, it may be useful to be familiar with their names. Note that each complex pumps protons from the mitochondrial matrix into the intermembrane space—with the exception of complex II.
Complex I: NADH-CoQ oxidoreductase
Complex II: Succinate-CoQ oxidoreductase
Complex III: CoQH2-cytochrome C oxidoreductase
Complex III is also the site of the Q cycle.
Complex IV: Cytochrome C oxidase
Here, cytochrome C is oxidized and an oxygen molecule is reduced, resulting in the formation of two water molecules.
Figure: The first four complexes of the electron transport chain.
The oxidation-reduction reactions catalyzed by the complexes within the electron transport chain must be strictly monitored for the release of highly destructive free radical species. If the mitochondria or cell is unable to combat radical oxygen species with antioxidants, the mitochondria may cease to function or the cell may die. This is a phenomenon known as oxidative stress, and can be a pathway through which the cell executes its own death (apoptosis).
b) The electrochemical gradient
Three of the four complexes of the electron transport chain pump protons into the intermembrane space. These protons serve to acidify the intermembrane space, creating the electrochemical gradient, which is critical for energy production.
The gradient is electrical because the protons carry a positive charge. Thus, as more protons are pumped into the intermembrane space, it becomes more and more positively charged. The gradient is also chemical because the increase in the number of protons in the intermembrane space also results in a lower pH. Recall that hydrogen ions are protons and a high concentration of them characterizes a low pH. This electrochemical gradient is also commonly referred to as the proton-motive force.
It might be worth memorizing the number of protons that are pumped into the intermembrane space for each molecule of NADH or FADH₂. NADH either directly or indirectly donates electrons to complexes I, III, and IV. Thus, for each NADH molecule, 10 protons are pumped into the intermembrane space (four protons at complex I, four protons at complex III, and two protons at complex IV). FADH₂ either directly or indirectly donates electrons to complexes II, III, and IV. Thus, only six protons are pumped into the intermembrane space for each FADH₂ molecule (none at complex II, four at complex III, and two at complex IV).
The electrochemical gradient is essential to produce ATP through ATP synthase. This is why it is so crucial for both the outer and inner membranes to be impermeable to ion exchange: if H+ ions were able to leak from either membrane, the gradient would rapidly dissipate.
c) Oxidative phosphorylation
The protons making up the electrochemical gradient are used to make ATP through a process known as oxidative phosphorylation. As the name implies, this process transfers energy that was released through the oxidation of NADH and FADH₂ into a new bond: one between ADP and an inorganic phosphate molecule. This results in the production of ATP.
Spanning the inner mitochondrial membrane is the enzyme ATP synthase, sometimes referred to as complex V. The enzyme itself is composed of two components. The F₀ component serves as a channel through which hydrogen ions in the inner mitochondrial space flow into the mitochondrial matrix. This flow of protons is also referred to as chemiosmosis.
The F₁ component uses the energy released by the gradient to create ATP via the phosphorylation of ADP. This phosphorylation is accomplished through conformational changes induced by the spinning of the F₁ component—leading to the F₁ component being known as a “molecular motor.”
Decoupling agents, or uncouplers, are molecules that inhibit the synthesis of ATP by destroying the proton gradient. Defects in proteins known as cytochromes commonly decouple this gradient.
Products of aerobic respiration
Now that we’ve covered the final step of aerobic respiration, let’s take a more macroscopic look at the process and discuss the net products. Recall that during glycolysis, pyruvate oxidation, and the citric acid cycle, ATP and electron carriers are produced. For the MCAT, you should know that for every NADH molecule that is generated, 2.5 ATP will be produced. Moreover, for every FADH₂ molecule that is generated, 1.5 ATP will be produced. After aerobic respiration, we will have a grand total of 32 ATP produced for every glucose molecule.
| Process | Net Products/Glucose Molecule |
|---|---|
2 ATP |
|
2 FADH2 → 3ATP 2GTP → 2ATP |
|
Figure: Each metabolic process uses a certain amount of electron carriers to synthesize a certain amount of ATP.
Note that oxidative phosphorylation is dependent on the presence of a proton gradient. Since the proton gradient is produced by the electron transport chain and the electron transport chain is dependent on the supply of NADH and FADH₂, the same energy dynamics that govern the production of these electron carriers dictates the function of oxidative phosphorylation. Thus, in high-energy states—when glucose is readily available, and glycolysis and the electron transport chain proceed—oxidative phosphorylation will also proceed. In low-energy states—when glucose is not readily available, and glycogenolysis or gluconeogenesis must proceed—the energy flux through oxidative phosphorylation decreases.
----
Part 6: Pentose phosphate pathway
The pentose phosphate pathway (PPP) ultimately produces ribose-5-phosphate: a molecule that is critical for nucleotide synthesis. This pathway takes place in the cytoplasm of the cell. While the MCAT will not test you on the specific details of nucleotide synthesis, it’s important to know the byproducts of the PPP and why they are important.
The PPP begins with the first step of glycolysis: the phosphorylation of glucose into glucose-6-phosphate. The first phase of the pathway, known as the oxidative phase, results in the production of two NADPH molecules and a molecule known as ribulose-5-phosphate. The second phase of the pathway, known as the non-oxidative phase, can result in multiple possible outcomes depending on the energy needs of the cell. The pathway can produce glycolytic intermediates to feed into glycolysis or ribose-5-phosphate to feed into nucleotide synthesis.
It’s important to note that the PPP does not produce any NADH—the type of electron carriers required for oxidative phosphorylation and ATP synthase. Rather, it produces NADPH, an important electron carrier in fatty acid synthesis.
----
Part 7: High-yield terms
Amylase: an enzyme that begins to hydrolyze large polymeric carbohydrates into monosaccharides and disaccharides
Glucagon: hormone secreted by α-cells when blood glucose levels are low
Insulin: hormone released by β-cells when blood glucose levels are high
Glycogenesis: pathway through which glucose is polymerized into glycogen
Glycogenolysis: pathway through which glycogen is deconstructed into glucose monomers
Glycolysis: metabolic process by which a glucose molecule is converted into two molecules of pyruvate
Phosphofructokinase 1: catalyzes the rate-limiting step of glycolysis
G3P dehydrogenase: an enzyme that catalyzes the reversible conversion of glyceraldehyde 3-phosphate into 1,3-bisphosphoglycerate, which yields two molecules of NADH per glucose molecule
Phosphoglycerate kinase: an enzyme that catalyzes the reversible conversion of 1,3-bisphosphoglycerate into 3-phosphoglycerate; generates one ATP per molecule of phosphoglycerate
Pyruvate kinase: an enzyme that catalyzes the irreversible conversion of phosphoenolpyruvate into pyruvate; generates one ATP per molecule of phosphoenolpyruvate
Lactic acid cycle: serves to regenerate NAD⁺ under anaerobic conditions, so glycolysis can continue
Pyruvate dehydrogenase: an enzyme catalyzes the irreversible conversion of pyruvate into acetyl-CoA and generates NADH and carbon dioxide as byproducts
Isocitrate dehydrogenase: an enzyme that catalyzes the rate-limiting step of the TCA cycle
Proton-motive force: the proton gradient established between the intermembrane space and the mitochondrial matrix
Chemiosmosis: the spontaneous flow of hydrogen ions through the F₀ component of ATP synthase
Decoupling agents: molecules that inhibit the synthesis of ATP by destroying the proton gradient present in the mitochondrion
Pentose phosphate pathway: produces NADPH that can be used in fatty acid synthesis and carbohydrate products that can be used in glycolysis or nucleotide synthesis
Looking for MCAT practice questions? Check out our proprietary MCAT Question Bank for 4000+ sample questions and eight practice tests covering every MCAT category.
Gain instant access to 4,000+ representative MCAT questions across all four sections to identify your weaknesses, bolster your strengths, and maximize your score. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.
----
Part 8: Passage-based questions and answers
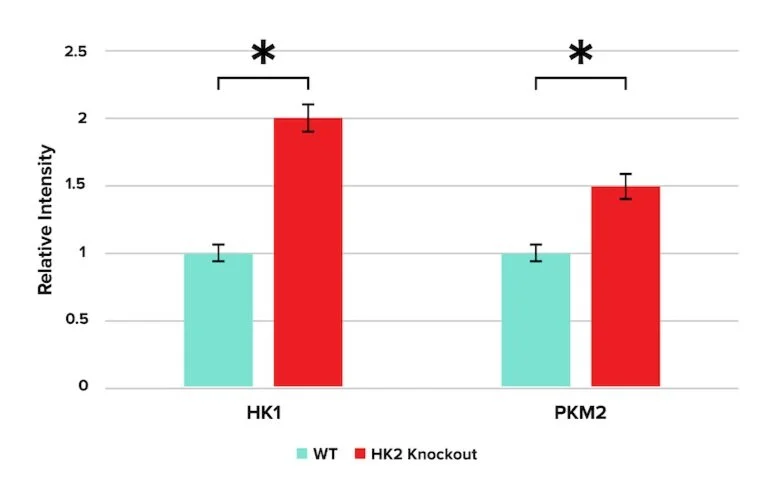
HK1 and HK2 are isozymes of hexokinase. HK1 is found in all mammalian tissues and is considered a “housekeeping enzyme” in physiological conditions. HK2, on the other hand, is present in fewer tissues under normal physiological conditions. HK2 is highly expressed in cancer cells. It has been found to play a key role in the “Warburg Effect,” in which certain cells preferentially metabolize glucose via glycolysis and fermentation under aerobic conditions. In the presence of oxygen, certain photoreceptors have been found to use glucose primarily for lactate production despite possessing abundant mitochondria and enzymes for oxidative phosphorylation. It remains unclear how photoreceptors balance aerobic fermentation and mitochondrial oxidative phosphorylation to regulate their survival.
To observe the effects of inhibiting aerobic fermentation on photoreceptor metabolic regulation, researchers selectively knock out the production of HK2 in mice. A Western blot was used to quantify changes in glycolytic enzymes, including hexokinase-1 (HK1) and pyruvate kinase isozyme M2 (PKM2). The results are shown in Figure 1.
Figure 1
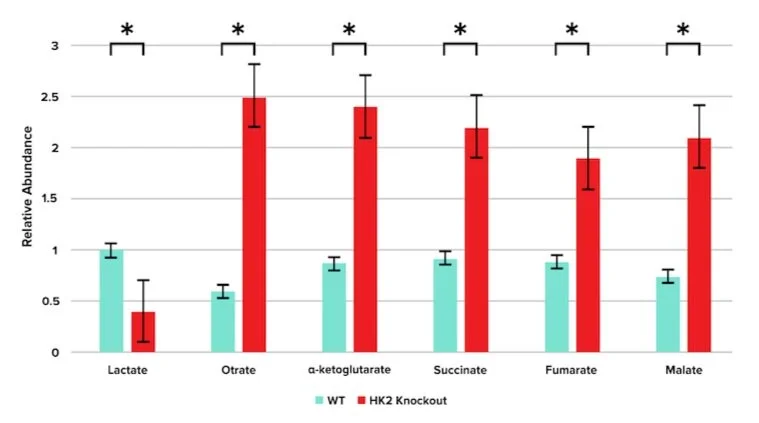
Researchers then performed mass spectrometry to profile changes in 13C-glucose-derived metabolites in fermentation and the TCA cycle. The results are shown in Figure 2.
Figure 2: Relative abundance of fermentation and TCA metabolites in WT and HK2-knockout mice.
CREATOR AND ATTRIBUTION PARTY: ZHANG, R., SHEN, W., DU, J. ET AL. SELECTIVE KNOCKDOWN OF HEXOKINASE 2 IN RODS LEADS TO AGE-RELATED PHOTORECEPTOR DEGENERATION AND RETINAL METABOLIC REMODELING. CELL DEATH DIS 11, 885 (2020). THE ARTICLE’S FULL TEXT IS AVAILABLE HERE: HTTPS://WWW.NATURE.COM/ARTICLES/S41419-020-03103-7. THE ARTICLE IS NOT COPYRIGHTED BY SHEMMASSIAN ACADEMIC CONSULTING. DISCLAIMER: SHEMMASSIAN ACADEMIC CONSULTING DOES NOT OWN THE PASSAGE PRESENTED HERE. CREATIVE COMMON LICENSE: HTTP://CREATIVECOMMONS.ORG/LICENSES/BY/4.0/. CHANGES WERE MADE TO ORIGINAL ARTICLE TO CREATE AN MCAT-STYLE PASSAGE.
Question 1: The data presented in Figure 1 indicate that downregulation of hexokinase-2 resulted in:
A) Apoptosis
B) Inhibition of glycolysis in all tissues
C) An upregulation of hexokinase-1
D) Increased production of ethanol
Question 2: Which of the following is the primary purpose of fermentation in humans?
A) Regeneration of NAD⁺
B) Regeneration of NADH
C) ATP production
D) Pyruvate production
Question 3: Why did the researchers seek to knock out hexokinase-2?
A) Hexokinase-2 is the isozyme that facilitates the Warburg Effect in photoreceptors
B) Hexokinase-2 is ubiquitously expressed in mammalian tissues
C) Hexokinase-1 is not found in humans
D) Hexokinase-2 is downregulated in cancer cells
Question 4: A researcher decides to selectively knock down isocitrate dehydrogenase in mice. What potential effect may this have?
A) Inhibition of fermentation
B) Increased levels of ATP
C) Inhibition of the citric acid cycle
D) Increased levels of succinate
Question 5: Based on the data presented in Figure 2, which of the following conclusions may be drawn?
A) Inhibition of hexokinase-2 promotes fermentation and the citric acid cycle
B) Inhibition of hexokinase-2 had no effect on the metabolic activity in photoreceptors
C) Inhibition of hexokinase-2 promotes fermentation but downregulates the citric acid cycle
D) Inhibition of hexokinase-2 downregulates fermentation but promotes the citric acid cycle
Answer key for passage-based questions
Answer choice C is correct. Figure 1, which displays the results of HK2 knockout, shows an increase in the levels of HK1 under HK2 knockout. This isozyme of hexokinase facilitates glycolysis in all tissues (choice B is incorrect). No apoptotic markers were mentioned in the figure (choice A is incorrect). Ethanol is produced by fermentation in yeast, not mammalian cells (choice D is incorrect).
Answer choice A is correct. During fermentation, pyruvate is converted into lactate and NADH is oxidized into NAD⁺ (choices B and D are incorrect). This production of NAD⁺ allows glycolysis to continue under anaerobic conditions. No ATP is produced during this process (choice C is incorrect).
Answer choice A is correct. The passage states that hexokinase-2 plays an important role in the Warburg Effect documented in certain photoreceptor cells (choice A is correct). Hexokinase-1, not hexokinase-2, is ubiquitously expressed in mammalian tissues (choice B is incorrect). Hexokinase-1 is found in humans (choice C is incorrect). Hexokinase-2 is regulated under normal physiological conditions but is highly expressed in cancer cells (choice D is incorrect).
Answer choice C is correct. Isocitrate dehydrogenase is the rate-limiting step of the citric acid cycle (choice C is correct). Inhibition of this enzyme would likely promote fermentation since the pyruvate formed by glycolysis would not be able to enter the TCA cycle and NADH would be rapidly consumed (choice A is incorrect). Glycolysis and fermentation are far less efficient at producing ATP than normal aerobic respiration. Succinate is a TCA cycle intermediate; a decrease in the activity of the TCA cycle would likely result in a decrease of succinate levels (choice D is incorrect).
Answer choice D is correct. Figure 2 shows that knockout mice had decreased lactate levels, indicating that fermentation is downregulated (choices A and C are incorrect). However, there are increased levels of citric acid cycle intermediates, indicating that the citric acid cycle is upregulated (choice D is correct).
----
Part 9: Standalone questions and answers
Question 1: Which type of bond does debranching enzyme hydrolyze?
A) α-1,4 glycosidic linkage
B) α-1,6 glycosidic linkage
C) β-1,4 glycosidic linkage
D) Both A and B
Question 2: Which of the following enzymes catalyzes an irreversible step in glycolysis?
I. Phosphofructokinase-1
II. Glyceraldehyde 3-Phosphate Dehydrogenase
III. Pyruvate Kinase
A) I only
B) II and III
C) I and III
D) I, II, and III
Question 3: An increase in the activity of pancreatic β-cells levels would likely lead to:
A) Direct activation of phosphofructokinase-1
B) Direct activation of phosphofructokinase-2
C) Direct activation of pyruvate kinase
D) Direct activation of debranching enzyme
Question 4: How much ATP would be produced by 10 NADH and 2 FADH₂ molecules?
A) 11
B) 7
C) 32
D) 28
Question 5: Which of the following accurately describes the action of complex I of the electron transport chain?
A) NAD⁺ is reduced to NADH and 4 protons are pumped into the intermembrane space
B) NAD⁺ is reduced to NADH and no proton pumping occurs
C) NADH is oxidized into NAD⁺ and 4 protons are pumped into the intermembrane space
D) NADH is oxidized into NAD⁺ and no proton pumping occurs
Question 6: Which of the following releases insulin into the blood when blood glucose levels are high?
I. α-cells
II. β-cells
III. δ-cells
A) I only
B) II only
C) III only
D) II and III
Question 7: What is the purpose of the F₀ component of ATP synthase?
A) It phosphorylates ADP molecules to form ATP
B) It regenerates NAD⁺
C) It catalyzes the reduction of oxygen into water
D) It serves as a channel for hydrogen ions to flow into the matrix
Question 8: Where in the cell does glycolysis occur?
A) The cytoplasm
B) The inner membrane of the mitochondria
C) The mitochondrial matrix
D) The intermembrane space
Answer key for practice questions
Answer choice D is correct. Debranching enzyme hydrolyzes the α-1,4 glycosidic linkage at a branch point and the α-1,6 glycosidic linkage between the linear chain and branched glucose molecule (choice C is incorrect).
Answer choice C is correct. Hexokinase, phosphofructokinase-1, and pyruvate kinase catalyze the three irreversible steps for glycolysis (choices I and III are correct). Glyceraldehyde 3-phosphate dehydrogenase catalyzes a reversible step of glycolysis (choice II is incorrect).
Answer choice B is correct. Pancreatic β-cells produce insulin. Insulin directly activates phosphofructokinase-2 (choice B is correct). This indirectly leads to an activation of phosphofructokinase-1 via the products of the reaction catalyzed by phosphofructokinase-2 (choice A is incorrect). Insulin does not activate pyruvate kinase (choice C is incorrect). Insulin promotes glycogen synthesis, and so would neither directly nor indirectly activate the debranching enzyme (choice D is incorrect).
Answer choice D is correct. For every molecule of NADH, 2.5 ATP is eventually produced. For every molecule of FADH₂, 1.5 ATP will be produced. 10 NADH molecules would result in 25 ATP, and 2 FADH₂ would result in 3 ATP. This gives us a total of 28 ATP (choices A, B, and C are incorrect).
Answer choice C is correct. At complex I, NADH is oxidized into NAD⁺ (choices A and B are incorrect). This results in four hydrogen ions being pumped into the intermembrane space (choice D is incorrect). While it is not necessary to memorize the action and effects of each complex of the electron transport chain, it is critical to know that the electron transport chain results in the oxidation of electron carriers and the pumping of hydrogen ions into the intermembrane space of the mitochondria.
Answer choice B is correct. Insulin is released by β-cells (choice II is correct). Glucagon is released by α-cells (choice I is incorrect). Somatostatin is released by δ-cells (choice III is incorrect).
Answer choice D is correct. The F₀ component serves as a channel through which hydrogen ions present in the inner mitochondrial space can flow back into the matrix (choice D is correct). This flow is the natural result of the chemiosmotic gradient. The F₀ component does not phosphorylate ADP molecules to form ATP; this function is performed by the F₁ component (choice A is incorrect). NAD⁺ is regenerated by lactate dehydrogenase during lactic acid fermentation (choice B is incorrect). Oxygen is reduced into water at complex IV of the electron transport chain (choice C is incorrect).
Answer choice A is correct. Glycolysis occurs in the cytoplasm (choice A is correct). The electron transport chain occurs along the inner membrane of the mitochondria (choice B is incorrect). Pyruvate oxidation and the citric acid cycle occur in the mitochondrial matrix (choice C is incorrect). The intermembrane space is where hydrogen ions are pumped during the electron transport chain, resulting in its acidification (choice D is incorrect).