Lipids and Membranes for the MCAT: Everything You Need to Know
Master MCAT lipids and membranes with clear explanations of phospholipids, membrane fluidity, transport, and practice questions with detailed answers.
(Note: This guide is part of our MCAT Biochemistry series .)
Part 1: Introduction to lipids and membranes
Part 2: Lipid structures
a) Insolubility of lipids
b) Signaling lipids
c) Structural lipids
Part 3: Cell membranes and components
a) Phospholipid bilayers
b) The fluid mosaic model
c) Major components of the cell membrane
d) Transporters
Part 4: High-yield terms
Part 5: Passage-based questions and answers
Part 6: Standalone questions and answers
----
Part 1: Introduction
In the early twentieth century, one of the most common therapies to control seizures in pediatric patients suffering from epilepsy was not a medication but a diet. Minimizing carbohydrates while consuming more fat seemed to alleviate seizures while still providing children the nutrients they needed to properly develop.
Fats, also known as lipids, are relatively simple molecules responsible for a variety of functions in our body, including energy storage and transmitting signals. The diversity of roles that lipids hold often makes them a challenging topic for students during their MCAT prep. Lipids are everywhere in your body—from hormones, cellular structures, and more. Indeed, they are a likely topic to show up on your exam.
In this guide, we will explain everything you need to know about lipids to be successful on the MCAT. At the end, we’ll include some practice passages and discrete questions so you can apply your knowledge in the exact context the AAMC will on test day.
Let’s get started!
----
Part 2: Lipid structures
a) Insolubility of lipids
Carbohydrates are characterized by their 1:2:1 ratio of carbon, hydrogen, and oxygen. Amino acids and proteins possess distinctive amine and carboxyl functional groups. Lipids are characterized by their hydrophobic alkyl structures and are primarily composed of carbon and hydrogen atoms.
Below we have testosterone, a signaling lipid, and a triglyceride, an energy-storing lipid.
Figure: Testosterone and a triglyceride.
Although they may look very different, they are both hydrophobic lipids. The hydrocarbon ring structure of testosterone and the hydrocarbon tail of the triglyceride will cause the molecules to be nonpolar and insoluble in polar solvents (such as water). This is a unifying feature of all lipids and has special implications for how lipids are used in the body.
Due to their nonpolar properties, lipids have assumed many different functions within organisms. All cell membranes are composed of lipids (as we will discuss), and many animals use lipids as a form of energy storage. Waxes are protective secretions that serve as a waterproofing compound for many plants and animals. The structure of waxes is characterized by long, alkane chains.
b) Signaling lipids
Signaling lipids are specialized lipids involved in signal transduction pathways, the passing of information between and within cells. These signaling lipids are further divided into two categories: steroids and fat-soluble vitamins.
Steroids are characterized by their four-ring structure, which includes three cyclohexanes and one cyclopentane. As you’d expect, these hydrocarbon rings make steroids nonpolar. The most commonly tested steroid on the MCAT, cholesterol, is one you should be very familiar with. It plays an integral role in our cell membranes (more on this later) and is a precursor for many molecules, including steroid hormones. Steroid hormones are specially secreted by endocrine glands and act as hormones. You can find more information about steroid hormones in our guide on the endocrine system.
Figure: The cyclic backbone of steroids.
Testosterone, the hormone we mentioned earlier, is just one of the many steroid hormones used by your body. Take another look at testosterone’s structure and see if you can identify the rings that characterize steroids.
Terpenes and terpenoids are aromatic secretions following the formula (C₅H₈)₁₁. Terpenes are typically rich in double bonds between carbons, allowing these molecules to undergo cyclization reactions. Squalene is an especially important terpene molecule as it is the biological precursor of steroids in the human body, including cholesterol.
Prostaglandins are an additional class of hormones derived from lipids. In contrast to steroid hormones, which often use cholesterol as a precursor, prostaglandins are derived from arachidonic acid. Prostaglandins contain at least one five-carbon ring.
Figure: An example of a prostaglandin.
Vitamins are nutrients that must be acquired through the diet. They often function as cofactors for enzymes. They can be divided into two categories: water-soluble vitamins and fat-soluble vitamins.
When in excess, water-soluble vitamins will be excreted in the urine, whereas fat-soluble vitamins will be stored in fat tissue. For the MCAT, you should know that vitamins B and C are water-soluble, while vitamins A, D, E, and K are fat-soluble.
c) Structural lipids
Like cholesterol, structural lipids play a crucial role in the cell membrane. These lipids are amphipathic, which simply means they have hydrophobic and hydrophilic regions on the same molecule. Their hydrophobic region is a nonpolar tail (typically composed of alkyl hydrocarbons), while the hydrophilic region is a polar head (such as a phosphate or glycerol group).
Figure: An example of a structural lipid with a hydrophilic head and two hydrophobic tails.
You may recall similar-sounding words with quite different meanings. For instance, an amphoteric compound can react as an acid or a base, and an amphiprotic substance can donate or receive a proton. It’s important not to get these terms confused!
For the MCAT, the two main structural lipids you should be familiar with are phospholipids and sphingolipids.
Phospholipids, or phosphatides, are the primary component of the phospholipid bilayer of the cell membrane. They contain a hydrophilic, polar phosphate head group joined through an ester linkage to a hydrophobic nonpolar fatty acid tail. An important property to keep in mind of these lipids is their degree of saturation. In this context, saturation refers to the number of single bonds a carbon atom has with other molecules.
Saturated fatty acid tails only have single bonds, so every carbon atom is bonded to four other atoms. These fully saturated tails form van der Waals interactions with other saturated fatty acid tails around them. Due to the symmetric, orderly nature of these alkyl chains, they can more easily form a cohesive structure and tend to be solid at room temperature.
Figure: An example of a saturated and unsaturated fatty acid.
Unsaturated fatty acid tails, on the other hand, contain one or more double bonds. These double bonds introduce “kinks” in the structure of the alkyl chain. This makes them less likely to stack and solidify. Thus, at room temperature, unsaturated fatty acids tend to be found in liquid form.
Why is this distinction important? As we will later discuss, the saturation of these fatty acid tails impacts the fluidity of the cell membrane. In general, unsaturated fatty acids are more prevalent in fluid regions of the cell membrane compared to saturated fatty acids.
Glycerophospholipids are an important type of phospholipids. Instead of a single phosphate head group, these contain a glycerol backbone that forms ester linkages to two fatty acids and a polar head group. Sphingolipids have a similar structure to glycerophospholipids—but instead of glycerol, they contain a sphingosine backbone in addition to the typical polar head group and nonpolar fatty acid tail.
Triglycerides, also known as triacylglycerols, are an additional group of lipids, which are composed of a glycerol head group and three fatty acid tails. They are primarily used for energy storage in specialized cells called adipocytes, and feature prominently in metabolism.
----
Part 3: Cell membranes and components
a) Phospholipid bilayers
As we've discussed, phospholipids are amphipathic—meaning they possess both a polar and nonpolar region on the same molecule. When placed in polar solutions—such as the aqueous environment of the body—these structures will assume some interesting shapes to minimize the free energy of the interactions.
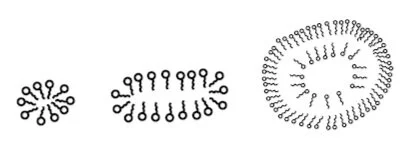
Since water is polar, the lipid’s hydrophobic regions will aggregate while the hydrophilic regions will interact with the solvent. This allows for structures like micelles to form. Micelles are spherical structures composed of a single layer of fatty acids arranged in their lowest energy state. The hydrophobic tails are bunched together on the interior of the bilayer, while the hydrophilic heads shield them from the polar environment. Bicelles are similar to micelles, in that they are lipids arranged in their lowest energy state. However, they are more disk-shaped and contain a planar region. Both micelles and bicelles are used in research to model the cell membrane, but bicelles tend to be favored as they most closely mimic natural cell structure.
A cell membrane sees phospholipids in yet another arrangement: a bilayer. As the name suggests, a bilayer is an arrangement of two layers of phospholipids. In a bilayer, the polar heads on the outer layer of phospholipids continue to interact with the aqueous environment, while the polar heads of the inner layer of phospholipids are oriented in the opposite direction to interact with the cytosol. The alkyl tails of both layers of phospholipid are left to create their own hydrophobic interactions and remain protected by the hydrophilic heads.
Figure: From left to right: a micelle, a bicelle, and a liposome. A liposome is an empty vesicle enclosed by a bilayer, and is heavily used in experimental design.
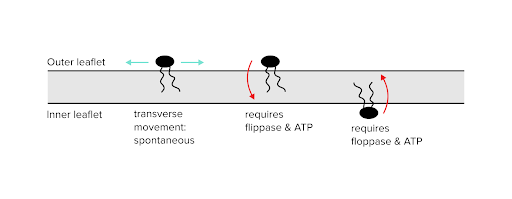
Phospholipids may need to be moved through the cell membrane or moved between either layer of a lipid bilayer. The random movement of phospholipids within a single layer can occur spontaneously and will occur over long timescales. The same can be said of switching phospholipids between an outer and inner bilayer.
To speed up the process of switching phospholipids between layers (or “leaflets”), special enzymes can be used. Flippases move a phospholipid from an outer leaflet to an inner leaflet. Floppases move a phospholipid from an inner leaflet to an outer leaflet. Since these are energetically demanding processes, ATP is required for both of these enzymes to function.
Figure: Dynamics between inner and outer leaflets
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.
b) The fluid mosaic model
While phospholipids are the primary component of the cell membrane, the cell membrane contains additional key components, including signaling carbohydrates, transmembrane proteins, and more. The fluid mosaic model describes the structure of these additional components within the “sea” of relatively fluid phospholipids that make up the bilayer. Carbohydrates, proteins, and less fluid lipids will aggregate to create “rafts” that are generally free to migrate around the surface of the membrane.
c) Major components of the cell membrane
Cholesterol
In addition to being a precursor for steroids, cholesterol plays a critical role in maintaining the cell membrane’s fluidity and stability. Remarkably, cholesterol can exhibit different behaviors depending on environmental conditions. At high temperatures, cholesterol limits the movement of phospholipids in the bilayer, which prevents the membrane from becoming overly fluid and losing its shape. At low temperatures, the rigid structure of cholesterol serves to disrupt the van der Waals interactions between fatty acid tails. This prevents the membrane from becoming too stiff and prone to breakage.
Signaling Carbohydrates
Recall that carbohydrates tend to be hydrophilic structures. They may also be attached to proteins on a cell’s extracellular surface, to facilitate intercellular signaling.
Carbohydrates can play crucial roles in signaling and immune recognition. For example, the antigens that characterize blood groups (ABO) are sphingolipids, which have different carbohydrate sequences.
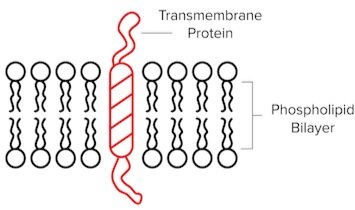
Transmembrane Proteins
In the cell membrane, transmembrane proteins span the entire membrane thanks to a region of hydrophobic amino acids. They often serve as channels that can facilitate transport or receptors that are involved in signal transduction pathways. G-protein coupled receptors (GPCRs) are just one example of transmembrane proteins you may frequently encounter.
Figure: A “one-pass” transmembrane protein that contains one hydrophobic region.
Transmembrane channels can be further classified into subtypes based on their method of action. Ligand-gated ion channels are channels that allow ions to pass into or out of the cell depending on the binding of a ligand, or signaling molecule, to a receptor. Just as signaling molecules bind to GPCRs to initiate a signal transduction pathway, signaling molecules may bind to ion channels to induce its opening and allow ions to flow.
Voltage-gated ion channels are channels that allow ions into or out of the cell based on the charge present in the cell membrane. One example is voltage-gated ion channels that open in response to depolarization during an action potential, allowing sodium ions to flow inside the cell. (For more information on action potentials, be sure to refer to our guide on the nervous system.)
Membrane-associated proteins
Additional proteins may link to, or associate with, the polar heads of phospholipids. While they do not span the internal, hydrophobic region of the lipid bilayer, they may still serve important roles in cell signaling and transport. The receptor portion of a GPCR is one example of a membrane-associated protein.
d) Transporters
As their name suggests, transporters perform transport processes. These transporters are typically specialized transmembrane proteins that move molecules into or out of the cell.
These processes may be classified based on their energy usage. Passive transport occurs when a molecule moves from an area of high concentration to an area of low concentration and no energy is required. On the other hand, active transport occurs when a molecule is moving from an area of low concentration to an area of high concentration. This process requires energy.
For the MCAT, you should be able to identify and describe the transport processes that follow.
Passive Transport
Simple diffusion, facilitated diffusion, and osmosis are different types of passive transport. Simple diffusion is the passive transport of a molecule without the aid of a transporter, while facilitated diffusion uses a transporter to passively move a molecule across the cell membrane. The latter is useful when a molecule is not able to cross the semipermeable cell membrane.
Osmosis is a type of simple diffusion that refers to water’s movement from a region of low solute concentration to high solute concentration. This movement of water can result in hypotonic, hypertonic, or isotonic conditions within the cell. In human cells, water molecules require the presence of aquaporins to pass through the cell membrane. Aquaporins are transmembrane channels that provide a polar environment for water molecules to pass through.
Active Transport
As we’ve mentioned, active transport processes are nonspontaneous and require energy. However, the exact sources of this energy may vary. Primary active transport processes acquire their energy through ATP hydrolysis. An example of this process can be seen with the sodium-potassium pump (also known as NaᐩKᐩ ATPase), which moves sodium and potassium ions using ATP. Along with passive transporters known as “leak channels,” the NaᐩKᐩ ATPase maintains sodium and potassium gradients and the cell membrane potential.
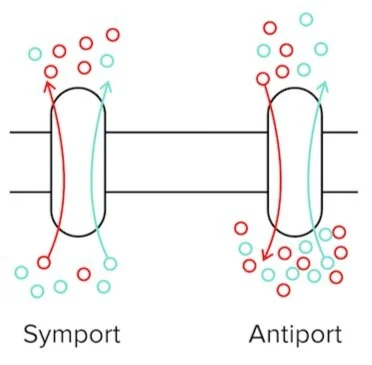
Secondary active transport processes power the unfavorable movement of a solute, such as an ion, against its concentration gradient by simultaneously moving another solute (favorably) down its gradient. Symport refers to a form of active transport in which these particles are both moving in the same direction. Antiport is another form of active transport in which they are moving in opposite directions.
Figure: A symporter and an antiporter. The blue solute is moving favorably down its concentration gradient, while the red solute must move against its concentration gradient.
----
Part 4: High-yield terms
Signaling lipids: specialized lipids involved in signal transduction pathways, or the passing of information between and within cells; include steroids and fat-soluble vitamins
Amphipathic: referring to the presence of both hydrophobic and hydrophilic regions on the same molecule
Phospholipids: the primary component of the phospholipid bilayer of the cell; contain a hydrophilic, polar phosphate head group joined through an ester linkage to a hydrophobic nonpolar fatty acid tail
Triglycerides: lipids composed of a glycerol head group and three fatty acid tails; primarily used for energy storage and feature prominently in metabolism.
Micelles: spherical structures composed of a single layer of fatty acids arranged in their lowest energy state
Liposome: an empty spherical vesicle enclosed by a bilayer
Fluid mosaic model: describes the structure of cell membrane components within the “sea” of relatively fluid phospholipids that make up the bilayer
Passive transport: occurs when a molecule moves from an area of high concentration to an area of low concentration and no energy is required
Active transport: occurs when a molecule is moving from an area of low concentration to an area of high concentration and the process requires energy; can be further categorized into primary and secondary active transport
Looking for MCAT practice questions? Check out our proprietary MCAT Question Bank for 4000+ sample questions and eight practice tests covering every MCAT category.
Gain instant access to 4,000+ representative MCAT questions across all four sections to identify your weaknesses, bolster your strengths, and maximize your score. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.
----
Part 5: Passage-based questions and answers
ABCA7 is a member of the ATP binding cassette subfamily A (ABCA), a group of evolutionary conserved cellular transmembrane transport proteins. ABCA7 in particular is thought to play an important role in lipid homeostasis in immune cells.
CD1d is a non-classical major histocompatibility complex protein that is involved in the presentation of lipid antigens to invariant natural killer (iNK) T cells, a special subpopulation of T cells. The binding of CD1d to its receptors on iNK T cells not only activates the iNK T cells but also activates pro-inflammatory downstream pathways in the CD1d expressing cells. CD1d is present on a wide variety of immune cells including thymocytes, hepatocytes, macrophages, and splenocytes. Previous studies have shown that CD1d localizes in lipid raft microdomains on the surface of these cells and that disruption of lipid rafts prevents CD1d antigen presentation.
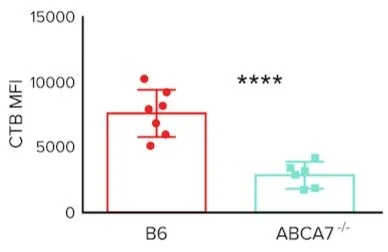
Based on prior knowledge of ABCA7’s role in immune cell lipid homeostasis, researchers have investigated the link between ABCA7 and CD1d antigen presentation. To do so, the researchers utilize a wild-type (B6) mouse line and an ABCA7 knockout (ABCA7-/-) mouse line. After isolating specific cells from these mice, the authors then stained for lipid rafts using either fluorescently-labeled cholera-toxin-B (CTB) or fluorescently labeled antibodies against caveolin-1. CTB is a protein that binds to components of lipid rafts while caveolin is a component of lipid rafts. Using these techniques, researchers generate the following data:
Figure 1. CTB mean fluorescence intensity (MFI) staining of splenocytes from B6 and ABCA7-/- mice
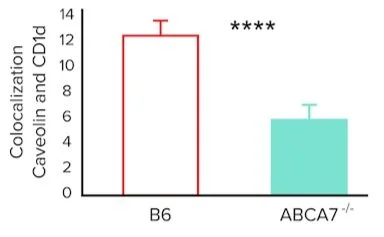
The researchers also want to investigate whether ABCA7 plays a role in lipid raft organization on the cell surface and if this affects CD1d antigen presentation. Their experiments generate the following data.
Figure 2. Colocalization (arbitrary units) of caveolin-1 and CD1d on the surface of splenocytes from B6 and ABCA7-/- mice
CREATOR AND ATTRIBUTION PARTY: NOWYHED, H., CHANDRA, S., KIOSSES, W. ET AL. ATP BINDING CASSETTE TRANSPORTER ABCA7 REGULATES NKT CELL DEVELOPMENT AND FUNCTION BY CONTROLLING CD1D EXPRESSION AND LIPID RAFT CONTENT. SCI REP 7, 40273 (2017). THE ARTICLE’S FULL TEXT IS AVAILABLE HERE: HTTPS://WWW.NATURE.COM/ARTICLES/SREP40273. THE ARTICLE IS NOT COPYRIGHTED BY SHEMMASSIAN ACADEMIC CONSULTING. DISCLAIMER: SHEMMASSIAN ACADEMIC CONSULTING DOES NOT OWN THE PASSAGE PRESENTED HERE. CREATIVE COMMON LICENSE: HTTP://CREATIVECOMMONS.ORG/LICENSES/BY/4.0/. CHANGES WERE MADE TO ORIGINAL ARTICLE TO CREATE AN MCAT-STYLE PASSAGE.
Question 1: Which of the following biomolecules can be found in the cell membrane?
I. Carbohydrates
II. Lipids
III. Proteins
IV. Nucleic Acids
A) II only
B) II and IV
C) I, II, IV
D) I, II, III
Question 2: Based on information in this passage, what important cellular biological role can lipid rafts directly affect?
A) Signal transduction
B) Cell-cell adhesion
C) Translation and transcription
D) Cell migration
Question 3: Based on the data presented in figure 1, how might the loss of ABCA7 function affect CD1d antigen presentation in splenocytes?
A) Loss of ABCA7 has no effect on CD1d antigen presentation
B) Loss of ABCA7 increases CD1d antigen presentation
C) Loss of ABCA7 decreases CD1d antigen presentation
D) Loss of ABCA7 changes the nature of antigens presented by CD1d
Question 4: Based on the results presented in Figure 2, what role does ABCA7 normally play in CD1d antigen presentation in the context of lipid raft organization?
A) ABCA7 promotes CD1d localization in lipid rafts on the cell surface
B) ABCA7 inhibits CD1d localization in lipid rafts on the cell surface
C) ABCA7 has no effect on CD1d localization in lipid rafts on the cell surface
D) ABCA7 affects the lipid composition of lipid rafts, promoting CD1d localization in lipid rafts on the cell surface
Question 5: Based on information presented in the passage, what role(s) does ABCA7 likely play in regulating CD1d antigen presentation on splenocytes?
I. ABCA7 helps transport lipid raft components to the cell surface, affecting CD1d antigen presentation
II. ABCA7 controls the composition of lipid rafts on the cell surface, affecting CD1d antigen presentation
III. ABCA7 influences expression of CD1d on the cell surface, affecting CD1d antigen presentation
A) I only
B) II only
C) I and II only
D) I, II, III
Answer key for passage-based questions:
1. Answer choice D is correct. Carbohydrates can be found on the surface of the cell membrane and are involved in immune recognition (I). Lipids, such as cholesterol and phospholipids, are the most plentiful component of the membrane (II). Proteins, such as transmembrane proteins, are also very prevalent in the membrane (III). Nucleic acids, on the other hand, are virtually absent (IV).
2. Answer choice A is correct. The passage states, “disruption of lipid rafts prevents CD1d antigen presentation.” Thus, we can conclude lipid rafts can directly affect signal transduction involved in antigen response (choice A is correct). In this case, lipid rafts affect signal transduction by preventing CD1d antigen presentation. The information presented in the passage is insufficient to support any other answer choices (choices B, C, and D are incorrect).
3. Answer choice C is correct. The data in figure 1 shows that in splenocytes, ABCA7 knockout decreases fluorescent staining. CTB, or cholera toxin B, binds to components of lipid rafts. This suggests that in splenocytes, ABCA7 knockout reduces the amount of lipid rafts present on the cell surface, thus suppressing CD1d antigen presentation by splenocytes (choice C is correct).
4. Answer choice A is correct. The data in figure 2 show that ABCA7 knockout decreases colocalization of caveolin-1 and CD1d. Since caveolin-1 is a component of lipid rafts, these data suggest that ABCA7 promotes CD1d localization in lipid rafts on the cell surface (choices B and C are incorrect). There is insufficient data to support the statement that ABCA7 affects the lipid composition of lipid rafts (choice D is incorrect).
5. Answer choice C is correct. The data in figure 1 shows decreased staining of CTB on the surface of ABCA7 knockout splenocytes compared to wild-type splenocytes. Since CTB binds components of lipid rafts, these data suggest that there is decreased expression of lipid raft components on the surface of ABCA7 knockout splenocytes. Thus, it is reasonable to conclude that ABCA7 helps transport lipid raft components to the cell surface (I). The data in figure 2 shows decreased colocalization of CD1d and caveolin-1 on the surface of ABCA7 knockout splenocytes compared to wild-type splenocytes. Since CD1d is a component of lipid rafts, it is reasonable to conclude that ABCA7 controls the composition of lipid rafts on the cell surface (II). No data on the expression of CD1d on the surface of splenocytes is shown; thus, statement III is incorrect.
----
Part 6: Standalone questions and answers
Question 1: Which of the following cellular metabolic processes would result in the highest net energy yield?
A) The glycolysis of two molecules of glucose
B) β-oxidation of an unsaturated 20 carbon fatty acid
C) β-oxidation of a saturated 20 carbon fatty acid
D) Both B and C
Question 2: Naᐩ/Kᐩ ATPase aids with or performs which type of transport process?
A) Primary active transport
B) Secondary active transport, by symporters
C) Secondary active transport, by antiports
D) Facilitated diffusion
Question 3: What is the fate of excess water-soluble vitamins in the body?
A) They are excreted in the urine
B) They are stored in adipose tissue
C) They are stored in the lymph nodes
D) They are converted into glucose and used to maintain blood glucose levels
Question 4: Which of the following statements is not true?
A) Cholesterol is a precursor for steroids
B) Cholesterol is a precursor for vitamin D
C) Cholesterol maintains the fluidity and stability of the cell membrane
D) Cholesterol aids in immune and intracellular recognition
Answer key for lipids practice questions:
Answer choice C is correct. Saturated fats lack double bonds. Because they are more reduced, the oxidation of a saturated fatty acid will yield more energy than an unsaturated fatty acid of the same length (choice B is correct). Glycolysis only yields a small amount of energy (choice A is incorrect).
Answer choice A is correct. The Naᐩ/Kᐩ-ATPase is the sodium-potassium pump, a common example of primary active transport (choice A is correct). Secondary active transport uses the energy of a particle moving down its concentration gradient to power the transport of one moving up its concentration gradient (choices B and C are incorrect). Facilitated diffusion is a type of passive transport (choice D is incorrect).
Answer choice A is correct. Excess water-soluble vitamins are excreted through the urine (choice A is correct). Excess fat-soluble vitamins are stored in adipose tissue (choice B is incorrect).
Answer choice D is correct. Carbohydrates, not cholesterol, can be found attached to proteins on the extracellular surface of a cell to aid in recognition (choice D is correct). The other statements regarding cholesterol are true (choices A, B, and C are incorrect).