Solutions and Gases for the MCAT: Everything You Need to Know
Learn essential MCAT topics covering solutions and gases, complete targeted practice problems, and review step-by-step answers to boost exam performance.
(Note: This guide is part of our MCAT General Chemistry series.)
Table of Contents
Part 1: Introduction to solutions and gases
Part 2: Solubility of ions
a) Solubility rules
b) Concentration units
c) Colligative properties
Part 3: Solubility of reactions
a) Common ion effect
b) Solubility equilibria
Part 4: Gas law equations
a) Ideal gas law
b) Ideal gas law derivatives
c) Partial pressure
Part 5: High-yield terms
Part 6: Passage-based questions and answers
Part 7: Standalone questions and answers
-----
Part 1: Introduction to solutions and gases
What happens as you stir sugar into a cup of coffee or salt in water? Perhaps predictably, the solid sugar or salt dissolves into its components: charged ions that are invisible to the naked eye. As ions become dissolved, they can change the chemistry of the aqueous solution they are in. Of course, this has special implications for compounds in biological systems, including acids and bases.
In this guide, we will cover everything you need to know about solutions and gases for the MCAT. You’ll run into some familiar variables, such as the equilibrium constant. However, this time it’ll be in the context of dissociation reactions. As you read through this article, challenge yourself to connect the topics discussed here with topics you’ve encountered during your MCAT prep.
Throughout this guide, there are several important terms and concepts marked in bold. At the end of the guide, there is also a practice passage and a set of questions for you to test your knowledge against.
Let’s begin!
-----
Part 2: Solubility of ions
a) Solubility rules
Solubility refers to the ability of a solute to dissolve in a particular solvent. For example, vitamins A, D, E, and K are considered fat-soluble vitamins because they dissolve in fat and are stored in adipose tissue. When the solute is in an equilibrium between its dissolved and undissolved state, the solution is considered to be saturated. Any additional solute added will be insoluble and form a precipitate, the solute’s undissolved solid form. These are referred to as precipitation reactions.
The solvent used and the temperature of a solution can impact the solubility of a substance. For instance, polar solvents (such as water) tend to dissolve other polar or ionic compounds. Nonpolar solvents (such as oil or fat) tend to dissolve other nonpolar compounds (such as vitamin A).
In the context of the MCAT, water is the most commonly seen solvent. The table below summarizes several solubility rules you should be familiar with for test day. Note that there are several exceptions to each of these solubility rules.
| Water Solubility Rules |
|---|
| Salts containing Group I elements (alkali metals) are soluble |
| Salts with the ammonium ion (NH4+) are soluble |
| Salts with the acetate ion (CH3COO-) are soluble |
| Salts with the nitrate ion (NO3-) are soluble |
|
Salts containing the sulfate ion (SO42-) are soluble
Exceptions: SrSO4, PbSO4, BaSO4, Ag2SO4, CaSO4 |
|
Sulfites (SO32-) are insoluble
Exceptions: Sulfites with Group I elements and ammonium |
|
Sulfides (S2-) are insoluble Exceptions: Sulfides with Group I elements and ammonium |
|
Halides are soluble (e.g., Iodine, Chlorine, Bromine) Exceptions: Fluoride and halides containing Pb2+, Ag+, and (Hg2)2+ |
|
Hydroxide salts with ammonium, Group I elements (alkali metals), and certain Group II elements (Ca2+, Sr2+, and Ba2+) are soluble All other hydroxide salts are insoluble |
|
Phosphates (PO43-) are insoluble Exceptions: Phosphates containing Group I elements and ammonium |
|
Carbonates (CO32-) are insoluble (e.g., CaCO3, FeCO3, and SrCO3) Exceptions: Carbonates containing Group I elements and ammonium |
This list of solubility rules may be fairly intimidating to memorize! However, as a general rule of thumb, ammonium, acetate, nitrate, and sulfate compounds are soluble. Sulfites, sulfides, calcium, and transition metal compounds tend to be insoluble.
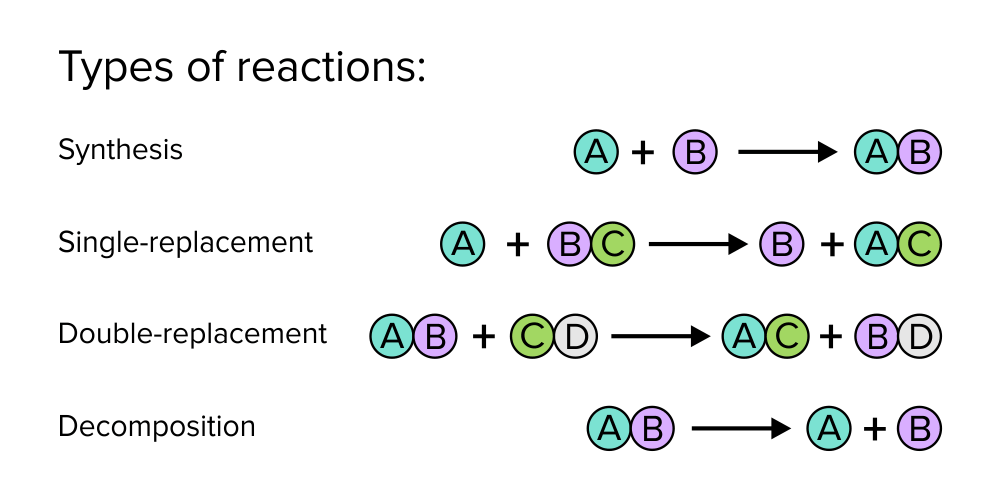
When compounds are dissolved in solution, new compounds can be formed through displacement or synthesis reactions. If such a reaction produces an insoluble compound, the resulting product will enter its solid form and precipitate from the reaction.
Figure: Common types of chemical reactions.
b) Concentration units
When a solution is made, a solute is said to be dissolved in a solvent. Thus, a solute tends to be a solid compound that is placed into a liquid-phase solvent. The quantity of solute that is placed within a volume of solvent is referred to as concentration. Solutions that have a smaller ratio of solute to solvent are described as dilute. Those that have a larger ratio are considered concentrated.
The concentration of a solution can be expressed in multiple ways. The most common unit used is molarity (M), which gives the number of moles of solute per liter of solution. Similarly, molality (m) gives the number of moles of solute per kilogram of solvent. Note that these two units have similar symbols but are very different!
In contrast, the units of osmoles refer to the number of moles of distinct solute particles. For example, for every 1 mole of potassium chloride (KCl) in a solution, there are 2 osmoles of solute particles. Osmolarity refers to the number of osmoles of solute per liter of solution, while osmolality refers to the osmoles of solute per kilogram of solvent.
Lastly, mole fraction gives the ratio of moles of one substance to moles of all substances within a solution or mixture.
| Unit | Symbol | Formula |
|---|---|---|
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.